Input interpretation

non-polar solvents | molar heat of combustion
Summary
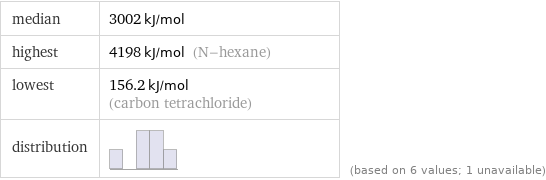
median | 3002 kJ/mol highest | 4198 kJ/mol (N-hexane) lowest | 156.2 kJ/mol (carbon tetrachloride) distribution | | (based on 6 values; 1 unavailable)
Entity with missing value
chloroform
Ranked values
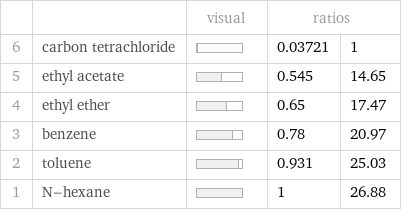
| | visual | ratios | 6 | carbon tetrachloride | | 0.03721 | 1 5 | ethyl acetate | | 0.545 | 14.65 4 | ethyl ether | | 0.65 | 17.47 3 | benzene | | 0.78 | 20.97 2 | toluene | | 0.931 | 25.03 1 | N-hexane | | 1 | 26.88
Molar heat of combustion rankings
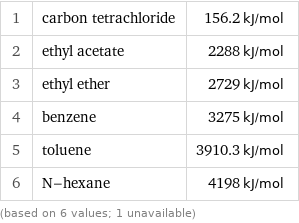
1 | carbon tetrachloride | 156.2 kJ/mol 2 | ethyl acetate | 2288 kJ/mol 3 | ethyl ether | 2729 kJ/mol 4 | benzene | 3275 kJ/mol 5 | toluene | 3910.3 kJ/mol 6 | N-hexane | 4198 kJ/mol (based on 6 values; 1 unavailable)
Unit conversions for median molar heat of combustion 3002 kJ/mol
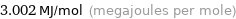
3.002 MJ/mol (megajoules per mole)
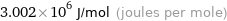
3.002×10^6 J/mol (joules per mole)
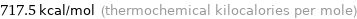
717.5 kcal/mol (thermochemical kilocalories per mole)

31.11 eV (molar electronvolts)