Input interpretation
potassium bromate
Chemical names and formulas
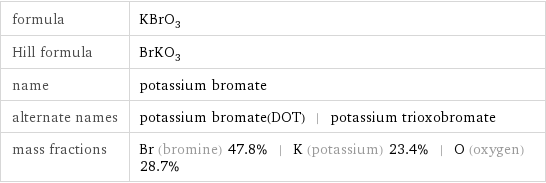
formula | KBrO_3 Hill formula | BrKO_3 name | potassium bromate alternate names | potassium bromate(DOT) | potassium trioxobromate mass fractions | Br (bromine) 47.8% | K (potassium) 23.4% | O (oxygen) 28.7%
Structure diagram
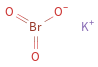
Structure diagram
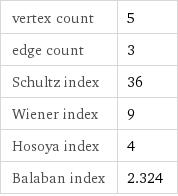
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
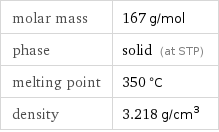
molar mass | 167 g/mol phase | solid (at STP) melting point | 350 °C density | 3.218 g/cm^3
Units

Solid properties (at STP)

density | 3.218 g/cm^3
Units

Thermodynamic properties
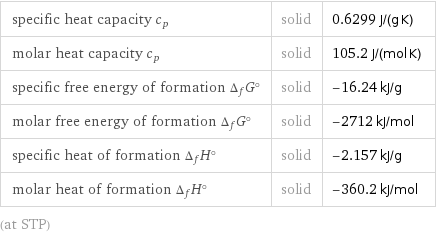
specific heat capacity c_p | solid | 0.6299 J/(g K) molar heat capacity c_p | solid | 105.2 J/(mol K) specific free energy of formation Δ_fG° | solid | -16.24 kJ/g molar free energy of formation Δ_fG° | solid | -2712 kJ/mol specific heat of formation Δ_fH° | solid | -2.157 kJ/g molar heat of formation Δ_fH° | solid | -360.2 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7758-01-2 PubChem CID number | 24444 SMILES identifier | [O-]Br(=O)=O.[K+] InChI identifier | InChI=1/BrHO3.K/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.K/q-1;m EU number | 231-829-8 Gmelin number | 15380 RTECS number | EF8725000 NSC number | 215200](../image_source/b9cbe2c9bd9d53ca94b299cf21311fdf.png)
CAS number | 7758-01-2 PubChem CID number | 24444 SMILES identifier | [O-]Br(=O)=O.[K+] InChI identifier | InChI=1/BrHO3.K/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fBrO3.K/q-1;m EU number | 231-829-8 Gmelin number | 15380 RTECS number | EF8725000 NSC number | 215200
NFPA label
NFPA label
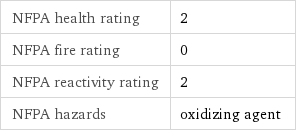
NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 2 NFPA hazards | oxidizing agent
Toxicity properties
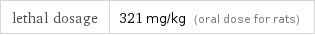
lethal dosage | 321 mg/kg (oral dose for rats)

probable lethal dose for man | 30 mL (milliliters) RTECS classes | tumorigen | mutagen | human data
Ion equivalents

K^+ (potassium cation) | 1 (BrO_3)^- (bromate anion) | 1