Input interpretation
lanthanum iodide
Chemical names and formulas
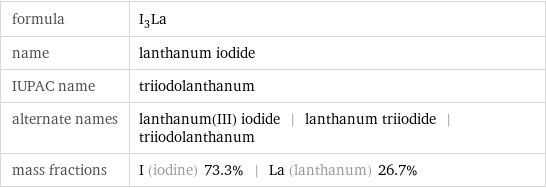
formula | I_3La name | lanthanum iodide IUPAC name | triiodolanthanum alternate names | lanthanum(III) iodide | lanthanum triiodide | triiodolanthanum mass fractions | I (iodine) 73.3% | La (lanthanum) 26.7%
Structure diagram
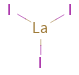
Structure diagram
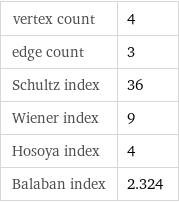
vertex count | 4 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
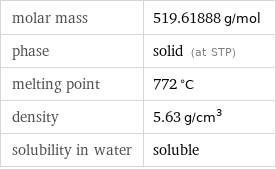
molar mass | 519.61888 g/mol phase | solid (at STP) melting point | 772 °C density | 5.63 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 5.63 g/cm^3
Units

Thermodynamic properties
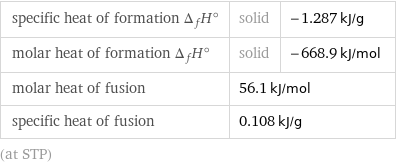
specific heat of formation Δ_fH° | solid | -1.287 kJ/g molar heat of formation Δ_fH° | solid | -668.9 kJ/mol molar heat of fusion | 56.1 kJ/mol | specific heat of fusion | 0.108 kJ/g | (at STP)
Chemical identifiers
I InChI identifier | InChI=1/3HI.La/h3*1H;/q;;;+3/p-3/f3I.La/h3*1h;/q3*-1;m MDL number | MFCD00049470](../image_source/9eb4d3d53d09dffb4b2f3e9405bb5587.png)
CAS number | 13813-22-4 PubChem CID number | 83743 PubChem SID number | 24865878 SMILES identifier | I[La](I)I InChI identifier | InChI=1/3HI.La/h3*1H;/q;;;+3/p-3/f3I.La/h3*1h;/q3*-1;m MDL number | MFCD00049470