Input interpretation

group 2 ions | molar mass
Summary
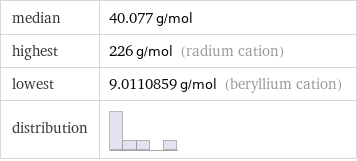
median | 40.077 g/mol highest | 226 g/mol (radium cation) lowest | 9.0110859 g/mol (beryllium cation) distribution |
Units

Ranked values
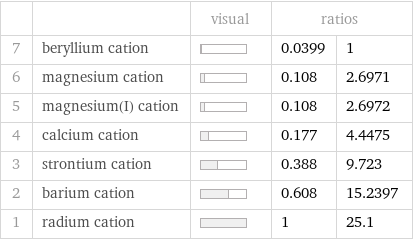
| | visual | ratios | 7 | beryllium cation | | 0.0399 | 1 6 | magnesium cation | | 0.108 | 2.6971 5 | magnesium(I) cation | | 0.108 | 2.6972 4 | calcium cation | | 0.177 | 4.4475 3 | strontium cation | | 0.388 | 9.723 2 | barium cation | | 0.608 | 15.2397 1 | radium cation | | 1 | 25.1
Molar mass rankings
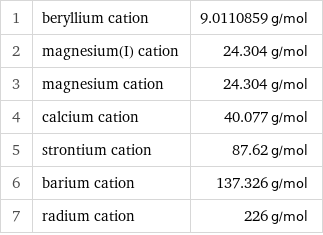
1 | beryllium cation | 9.0110859 g/mol 2 | magnesium(I) cation | 24.304 g/mol 3 | magnesium cation | 24.304 g/mol 4 | calcium cation | 40.077 g/mol 5 | strontium cation | 87.62 g/mol 6 | barium cation | 137.326 g/mol 7 | radium cation | 226 g/mol
Unit conversion for median molar mass 40.077 g/mol
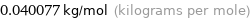
0.040077 kg/mol (kilograms per mole)
Comparisons for median molar mass 40.077 g/mol
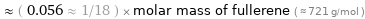
≈ ( 0.056 ≈ 1/18 ) × molar mass of fullerene ( ≈ 721 g/mol )
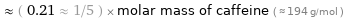
≈ ( 0.21 ≈ 1/5 ) × molar mass of caffeine ( ≈ 194 g/mol )
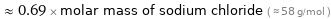
≈ 0.69 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
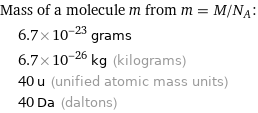
Mass of a molecule m from m = M/N_A: | 6.7×10^-23 grams | 6.7×10^-26 kg (kilograms) | 40 u (unified atomic mass units) | 40 Da (daltons)
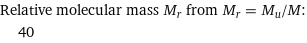
Relative molecular mass M_r from M_r = M_u/M: | 40