Input interpretation
silver ions
Members
silver(I) cation | silver(II) cation | diamminesilver(I) cation
Structure diagrams

Structure diagrams
General properties
![| silver(I) cation | silver(II) cation | diamminesilver(I) cation formula | Ag^+ | Ag^(2+) | ([Ag(NH_3)_2])^+ net ionic charge | +1 | +2 | +1 alternate names | silver(I) | silver(1+) | silver(II) | silver(2+) | diamminesilver(I) | diamminesilver(1+)](../image_source/ac5e0bafc666f0e6a5a841d777c083ba.png)
| silver(I) cation | silver(II) cation | diamminesilver(I) cation formula | Ag^+ | Ag^(2+) | ([Ag(NH_3)_2])^+ net ionic charge | +1 | +2 | +1 alternate names | silver(I) | silver(1+) | silver(II) | silver(2+) | diamminesilver(I) | diamminesilver(1+)
Ionic radii
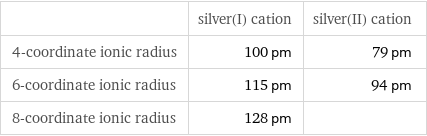
| silver(I) cation | silver(II) cation 4-coordinate ionic radius | 100 pm | 79 pm 6-coordinate ionic radius | 115 pm | 94 pm 8-coordinate ionic radius | 128 pm |
Units

Other properties
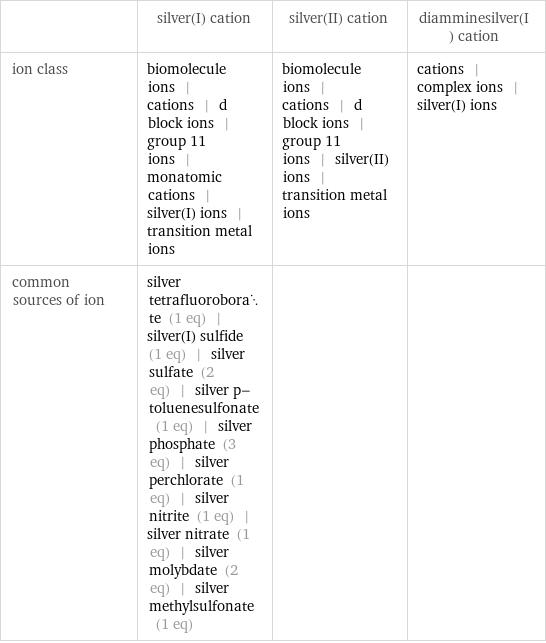
| silver(I) cation | silver(II) cation | diamminesilver(I) cation ion class | biomolecule ions | cations | d block ions | group 11 ions | monatomic cations | silver(I) ions | transition metal ions | biomolecule ions | cations | d block ions | group 11 ions | silver(II) ions | transition metal ions | cations | complex ions | silver(I) ions common sources of ion | silver tetrafluoroborate (1 eq) | silver(I) sulfide (1 eq) | silver sulfate (2 eq) | silver p-toluenesulfonate (1 eq) | silver phosphate (3 eq) | silver perchlorate (1 eq) | silver nitrite (1 eq) | silver nitrate (1 eq) | silver molybdate (2 eq) | silver methylsulfonate (1 eq) | |