Input interpretation

palladium (chemical element) | niobium (chemical element) | molybdenum (chemical element)
Periodic table location
Periodic table location
Images
Images
Basic elemental properties
![| palladium | niobium | molybdenum atomic symbol | Pd | Nb | Mo atomic number | 46 | 41 | 42 short electronic configuration | [Kr]4d^10 | [Kr]5s^14d^4 | [Kr]5s^14d^5 Aufbau diagram | 4d | 4d 5s | 4d 5s block | d | d | d group | 10 | 5 | 6 period | 5 | 5 | 5 atomic mass | 106.42 u | 92.90637 u | 95.95 u half-life | (stable) | (stable) | (stable)](../image_source/c44b3a3c72a2a529cfc8caa31b8207f4.png)
| palladium | niobium | molybdenum atomic symbol | Pd | Nb | Mo atomic number | 46 | 41 | 42 short electronic configuration | [Kr]4d^10 | [Kr]5s^14d^4 | [Kr]5s^14d^5 Aufbau diagram | 4d | 4d 5s | 4d 5s block | d | d | d group | 10 | 5 | 6 period | 5 | 5 | 5 atomic mass | 106.42 u | 92.90637 u | 95.95 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
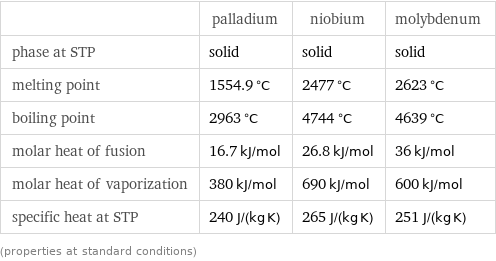
| palladium | niobium | molybdenum phase at STP | solid | solid | solid melting point | 1554.9 °C | 2477 °C | 2623 °C boiling point | 2963 °C | 4744 °C | 4639 °C molar heat of fusion | 16.7 kJ/mol | 26.8 kJ/mol | 36 kJ/mol molar heat of vaporization | 380 kJ/mol | 690 kJ/mol | 600 kJ/mol specific heat at STP | 240 J/(kg K) | 265 J/(kg K) | 251 J/(kg K) (properties at standard conditions)
Material properties
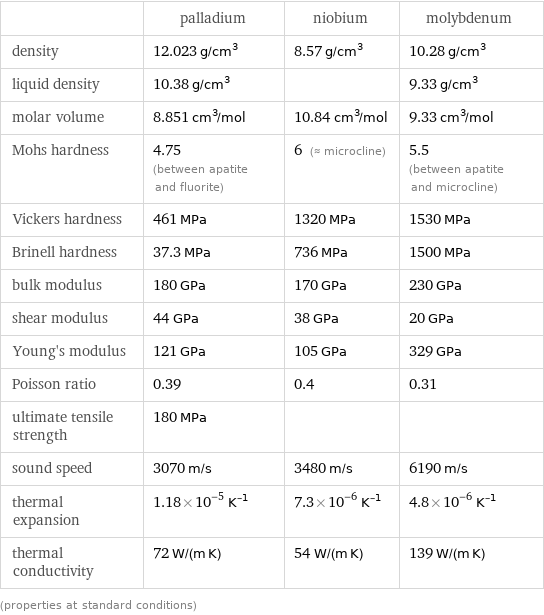
| palladium | niobium | molybdenum density | 12.023 g/cm^3 | 8.57 g/cm^3 | 10.28 g/cm^3 liquid density | 10.38 g/cm^3 | | 9.33 g/cm^3 molar volume | 8.851 cm^3/mol | 10.84 cm^3/mol | 9.33 cm^3/mol Mohs hardness | 4.75 (between apatite and fluorite) | 6 (≈ microcline) | 5.5 (between apatite and microcline) Vickers hardness | 461 MPa | 1320 MPa | 1530 MPa Brinell hardness | 37.3 MPa | 736 MPa | 1500 MPa bulk modulus | 180 GPa | 170 GPa | 230 GPa shear modulus | 44 GPa | 38 GPa | 20 GPa Young's modulus | 121 GPa | 105 GPa | 329 GPa Poisson ratio | 0.39 | 0.4 | 0.31 ultimate tensile strength | 180 MPa | | sound speed | 3070 m/s | 3480 m/s | 6190 m/s thermal expansion | 1.18×10^-5 K^(-1) | 7.3×10^-6 K^(-1) | 4.8×10^-6 K^(-1) thermal conductivity | 72 W/(m K) | 54 W/(m K) | 139 W/(m K) (properties at standard conditions)
Electromagnetic properties
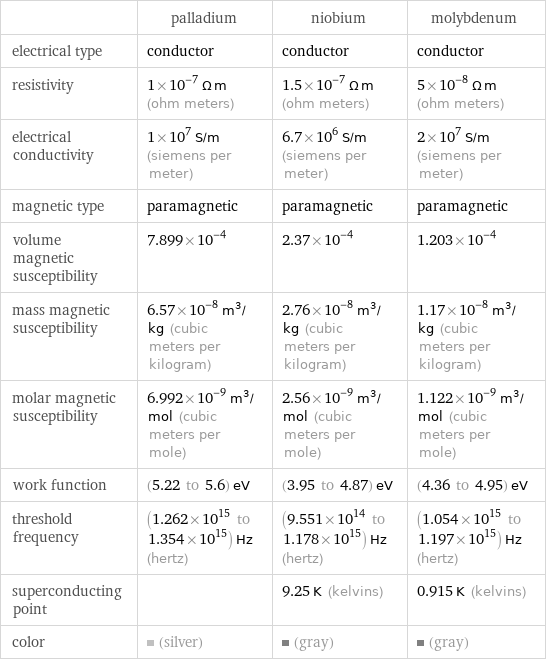
| palladium | niobium | molybdenum electrical type | conductor | conductor | conductor resistivity | 1×10^-7 Ω m (ohm meters) | 1.5×10^-7 Ω m (ohm meters) | 5×10^-8 Ω m (ohm meters) electrical conductivity | 1×10^7 S/m (siemens per meter) | 6.7×10^6 S/m (siemens per meter) | 2×10^7 S/m (siemens per meter) magnetic type | paramagnetic | paramagnetic | paramagnetic volume magnetic susceptibility | 7.899×10^-4 | 2.37×10^-4 | 1.203×10^-4 mass magnetic susceptibility | 6.57×10^-8 m^3/kg (cubic meters per kilogram) | 2.76×10^-8 m^3/kg (cubic meters per kilogram) | 1.17×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | 6.992×10^-9 m^3/mol (cubic meters per mole) | 2.56×10^-9 m^3/mol (cubic meters per mole) | 1.122×10^-9 m^3/mol (cubic meters per mole) work function | (5.22 to 5.6) eV | (3.95 to 4.87) eV | (4.36 to 4.95) eV threshold frequency | (1.262×10^15 to 1.354×10^15) Hz (hertz) | (9.551×10^14 to 1.178×10^15) Hz (hertz) | (1.054×10^15 to 1.197×10^15) Hz (hertz) superconducting point | | 9.25 K (kelvins) | 0.915 K (kelvins) color | (silver) | (gray) | (gray)
Reactivity
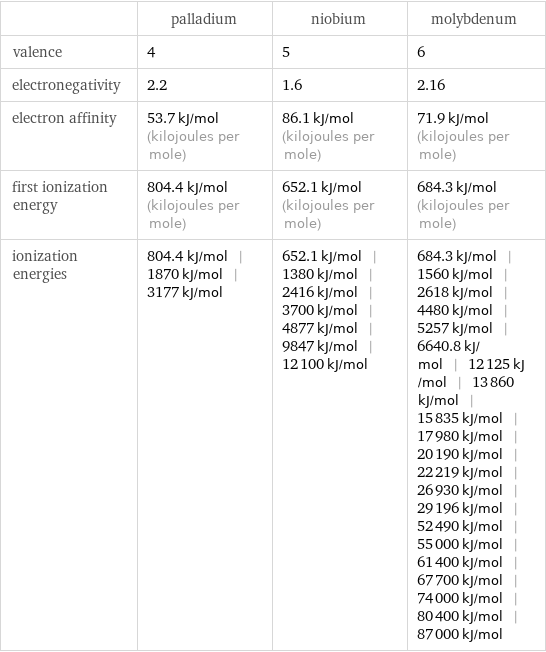
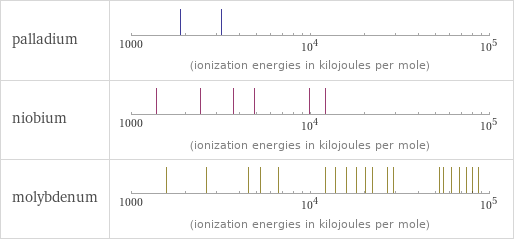
| palladium | niobium | molybdenum valence | 4 | 5 | 6 electronegativity | 2.2 | 1.6 | 2.16 electron affinity | 53.7 kJ/mol (kilojoules per mole) | 86.1 kJ/mol (kilojoules per mole) | 71.9 kJ/mol (kilojoules per mole) first ionization energy | 804.4 kJ/mol (kilojoules per mole) | 652.1 kJ/mol (kilojoules per mole) | 684.3 kJ/mol (kilojoules per mole) ionization energies | 804.4 kJ/mol | 1870 kJ/mol | 3177 kJ/mol | 652.1 kJ/mol | 1380 kJ/mol | 2416 kJ/mol | 3700 kJ/mol | 4877 kJ/mol | 9847 kJ/mol | 12100 kJ/mol | 684.3 kJ/mol | 1560 kJ/mol | 2618 kJ/mol | 4480 kJ/mol | 5257 kJ/mol | 6640.8 kJ/mol | 12125 kJ/mol | 13860 kJ/mol | 15835 kJ/mol | 17980 kJ/mol | 20190 kJ/mol | 22219 kJ/mol | 26930 kJ/mol | 29196 kJ/mol | 52490 kJ/mol | 55000 kJ/mol | 61400 kJ/mol | 67700 kJ/mol | 74000 kJ/mol | 80400 kJ/mol | 87000 kJ/mol
Reactivity
Atomic properties

| palladium | niobium | molybdenum term symbol | ^1S_0 | ^6D_(1/2) | ^7S_3 atomic radius | 140 pm | 145 pm | 145 pm covalent radius | 139 pm | 164 pm | 154 pm van der Waals radius | 163 pm | | (electronic ground state properties)
Abundances
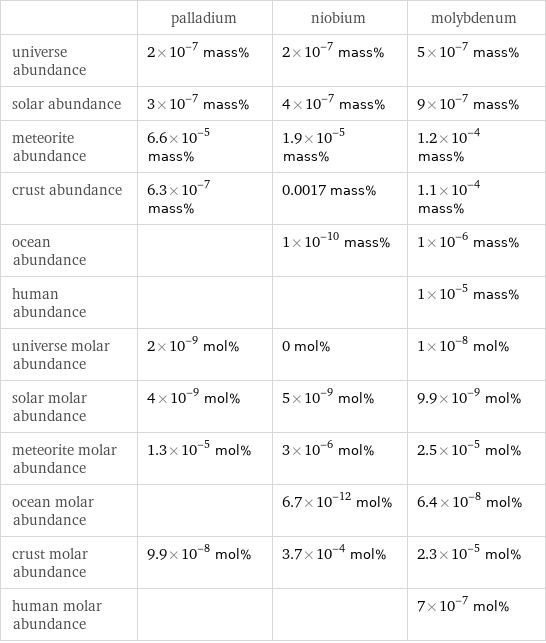
| palladium | niobium | molybdenum universe abundance | 2×10^-7 mass% | 2×10^-7 mass% | 5×10^-7 mass% solar abundance | 3×10^-7 mass% | 4×10^-7 mass% | 9×10^-7 mass% meteorite abundance | 6.6×10^-5 mass% | 1.9×10^-5 mass% | 1.2×10^-4 mass% crust abundance | 6.3×10^-7 mass% | 0.0017 mass% | 1.1×10^-4 mass% ocean abundance | | 1×10^-10 mass% | 1×10^-6 mass% human abundance | | | 1×10^-5 mass% universe molar abundance | 2×10^-9 mol% | 0 mol% | 1×10^-8 mol% solar molar abundance | 4×10^-9 mol% | 5×10^-9 mol% | 9.9×10^-9 mol% meteorite molar abundance | 1.3×10^-5 mol% | 3×10^-6 mol% | 2.5×10^-5 mol% ocean molar abundance | | 6.7×10^-12 mol% | 6.4×10^-8 mol% crust molar abundance | 9.9×10^-8 mol% | 3.7×10^-4 mol% | 2.3×10^-5 mol% human molar abundance | | | 7×10^-7 mol%