Input interpretation
chromium(III) oxide
Chemical names and formulas
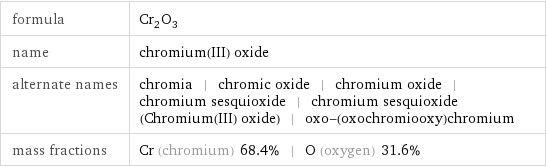
formula | Cr_2O_3 name | chromium(III) oxide alternate names | chromia | chromic oxide | chromium oxide | chromium sesquioxide | chromium sesquioxide (Chromium(III) oxide) | oxo-(oxochromiooxy)chromium mass fractions | Cr (chromium) 68.4% | O (oxygen) 31.6%
Structure diagram
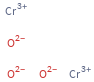
Structure diagram
Basic properties
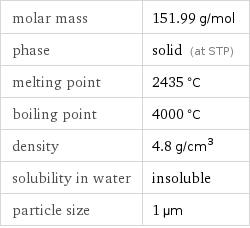
molar mass | 151.99 g/mol phase | solid (at STP) melting point | 2435 °C boiling point | 4000 °C density | 4.8 g/cm^3 solubility in water | insoluble particle size | 1 µm
Solid properties (at STP)

density | 4.8 g/cm^3 vapor pressure | 3 mmHg (at 2000 °C)
Units

Thermodynamic properties

specific heat capacity c_p | solid | 0.781 J/(g K) molar heat capacity c_p | solid | 118.7 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.962 kJ/g molar free energy of formation Δ_fG° | solid | -1058 kJ/mol specific heat of formation Δ_fH° | solid | -7.499 kJ/g molar heat of formation Δ_fH° | solid | -1140 kJ/mol specific entropy S° | solid | 0.5329 J/(g K) molar entropy S° | solid | 81 J/(mol K) molar heat of fusion | 125 kJ/mol | specific heat of fusion | 0.822 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1308-38-9 SMILES identifier | [Cr+3].[Cr+3].[O-2].[O-2].[O-2] RTECS number | GB6475000 MDL number | MFCD00010949](../image_source/231011a780e4c9aa254f2086c0d873a3.png)
CAS number | 1308-38-9 SMILES identifier | [Cr+3].[Cr+3].[O-2].[O-2].[O-2] RTECS number | GB6475000 MDL number | MFCD00010949
Toxicity properties
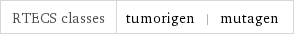
RTECS classes | tumorigen | mutagen