Input interpretation
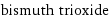
bismuth trioxide
Chemical names and formulas

formula | Bi_2O_3 name | bismuth trioxide IUPAC name | oxo-oxobismuthanyloxybismuthane alternate names | bismuth(III) oxide | bismuthous oxide | bismuth oxide | bismuth sesquioxide | bismuth yellow | dibismuth trioxide | keto-ketobismuthanyloxy-bismuthane | oxo-oxobismuthanyloxy-bismuthane | oxo-oxobismuthanyloxybismuthane mass fractions | Bi (bismuth) 89.7% | O (oxygen) 10.3%
Structure diagram

Structure diagram
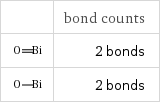
| bond counts | 2 bonds | 2 bonds
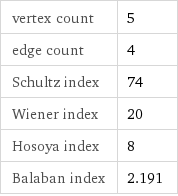
vertex count | 5 edge count | 4 Schultz index | 74 Wiener index | 20 Hosoya index | 8 Balaban index | 2.191
Basic properties
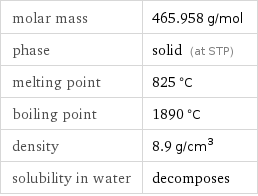
molar mass | 465.958 g/mol phase | solid (at STP) melting point | 825 °C boiling point | 1890 °C density | 8.9 g/cm^3 solubility in water | decomposes
Units

Solid properties (at STP)

density | 8.9 g/cm^3
Units

Thermodynamic properties
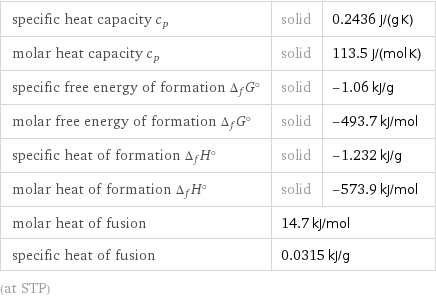
specific heat capacity c_p | solid | 0.2436 J/(g K) molar heat capacity c_p | solid | 113.5 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.06 kJ/g molar free energy of formation Δ_fG° | solid | -493.7 kJ/mol specific heat of formation Δ_fH° | solid | -1.232 kJ/g molar heat of formation Δ_fH° | solid | -573.9 kJ/mol molar heat of fusion | 14.7 kJ/mol | specific heat of fusion | 0.0315 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1304-76-3 PubChem CID number | 14776 PubChem SID number | 24852122 SMILES identifier | O=[Bi]O[Bi]=O InChI identifier | InChI=1/2Bi.3O/rBi2O3/c3-1-5-2-4 RTECS number | EB2984460 MDL number | MFCD00003462](../image_source/a5d36c87bd079d5b560a7594f7812394.png)
CAS number | 1304-76-3 PubChem CID number | 14776 PubChem SID number | 24852122 SMILES identifier | O=[Bi]O[Bi]=O InChI identifier | InChI=1/2Bi.3O/rBi2O3/c3-1-5-2-4 RTECS number | EB2984460 MDL number | MFCD00003462
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

odor | odorless