Input interpretation

polar protic solvents | Henry law constant
Summary

median | 0.6941 Pa m^3/mol highest | 4.479 Pa m^3/mol (acetic acid) lowest | 0.01692 Pa m^3/mol (formic acid) | (based on 7 values; 1 unavailable)
Entity with missing value
water
Ranked values
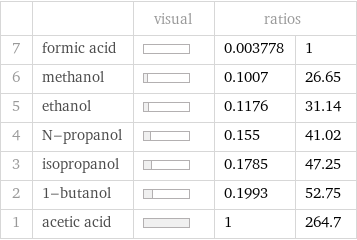
| | visual | ratios | 7 | formic acid | | 0.003778 | 1 6 | methanol | | 0.1007 | 26.65 5 | ethanol | | 0.1176 | 31.14 4 | N-propanol | | 0.155 | 41.02 3 | isopropanol | | 0.1785 | 47.25 2 | 1-butanol | | 0.1993 | 52.75 1 | acetic acid | | 1 | 264.7
Henry law constant rankings
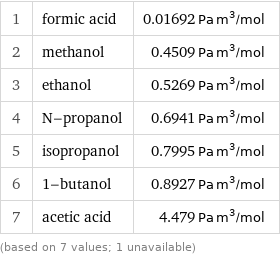
1 | formic acid | 0.01692 Pa m^3/mol 2 | methanol | 0.4509 Pa m^3/mol 3 | ethanol | 0.5269 Pa m^3/mol 4 | N-propanol | 0.6941 Pa m^3/mol 5 | isopropanol | 0.7995 Pa m^3/mol 6 | 1-butanol | 0.8927 Pa m^3/mol 7 | acetic acid | 4.479 Pa m^3/mol (based on 7 values; 1 unavailable)
Unit conversion for median Henry law constant 0.6941 Pa m^3/mol
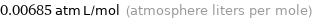
0.00685 atm L/mol (atmosphere liters per mole)