Input interpretation
L-tryptophan | L-serine
Chemical names and formulas
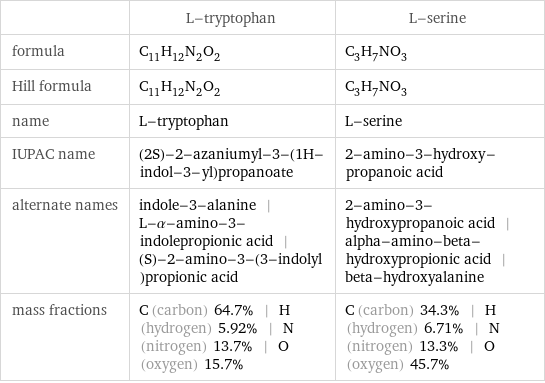
| L-tryptophan | L-serine formula | C_11H_12N_2O_2 | C_3H_7NO_3 Hill formula | C_11H_12N_2O_2 | C_3H_7NO_3 name | L-tryptophan | L-serine IUPAC name | (2S)-2-azaniumyl-3-(1H-indol-3-yl)propanoate | 2-amino-3-hydroxy-propanoic acid alternate names | indole-3-alanine | L-α-amino-3-indolepropionic acid | (S)-2-amino-3-(3-indolyl)propionic acid | 2-amino-3-hydroxypropanoic acid | alpha-amino-beta-hydroxypropionic acid | beta-hydroxyalanine mass fractions | C (carbon) 64.7% | H (hydrogen) 5.92% | N (nitrogen) 13.7% | O (oxygen) 15.7% | C (carbon) 34.3% | H (hydrogen) 6.71% | N (nitrogen) 13.3% | O (oxygen) 45.7%
Structure diagrams
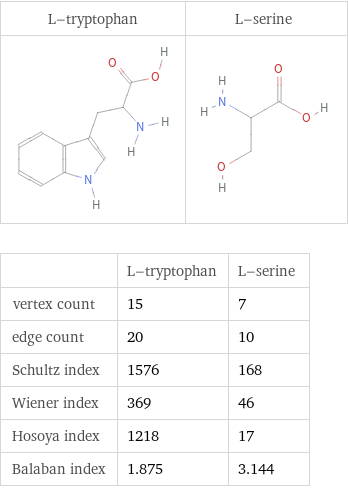
| L-tryptophan | L-serine vertex count | 15 | 7 edge count | 20 | 10 Schultz index | 1576 | 168 Wiener index | 369 | 46 Hosoya index | 1218 | 17 Balaban index | 1.875 | 3.144
3D structure
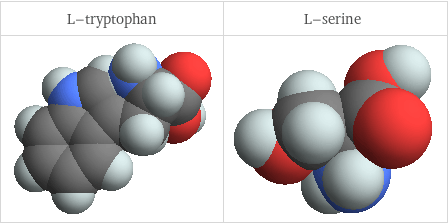
3D structure
Basic properties
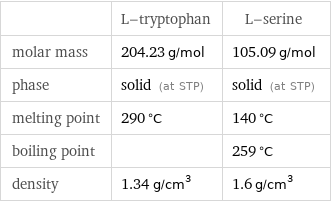
| L-tryptophan | L-serine molar mass | 204.23 g/mol | 105.09 g/mol phase | solid (at STP) | solid (at STP) melting point | 290 °C | 140 °C boiling point | | 259 °C density | 1.34 g/cm^3 | 1.6 g/cm^3
Units

Hydrophobicity and permeability properties
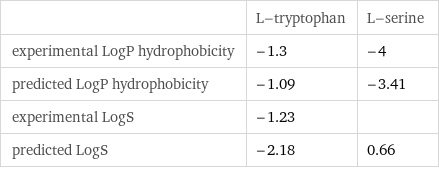
| L-tryptophan | L-serine experimental LogP hydrophobicity | -1.3 | -4 predicted LogP hydrophobicity | -1.09 | -3.41 experimental LogS | -1.23 | predicted LogS | -2.18 | 0.66
Amino acid properties
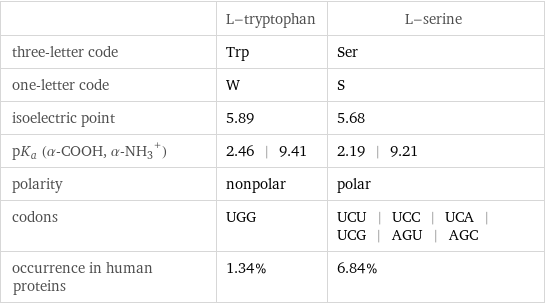
| L-tryptophan | L-serine three-letter code | Trp | Ser one-letter code | W | S isoelectric point | 5.89 | 5.68 pK_a (α-COOH, (α-NH_3)^+) | 2.46 | 9.41 | 2.19 | 9.21 polarity | nonpolar | polar codons | UGG | UCU | UCC | UCA | UCG | AGU | AGC occurrence in human proteins | 1.34% | 6.84%