Input interpretation
mercury(II) nitrate
Chemical names and formulas
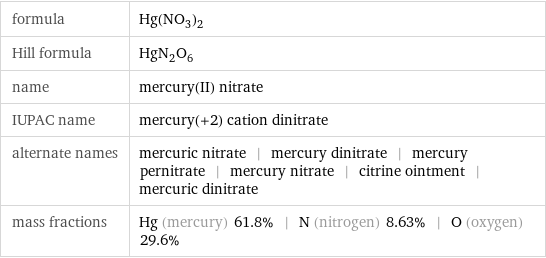
formula | Hg(NO_3)_2 Hill formula | HgN_2O_6 name | mercury(II) nitrate IUPAC name | mercury(+2) cation dinitrate alternate names | mercuric nitrate | mercury dinitrate | mercury pernitrate | mercury nitrate | citrine ointment | mercuric dinitrate mass fractions | Hg (mercury) 61.8% | N (nitrogen) 8.63% | O (oxygen) 29.6%
Structure diagram
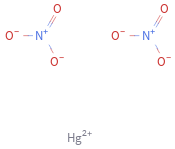
Structure diagram
Basic properties
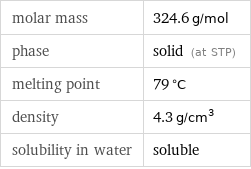
molar mass | 324.6 g/mol phase | solid (at STP) melting point | 79 °C density | 4.3 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
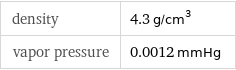
density | 4.3 g/cm^3 vapor pressure | 0.0012 mmHg
Units

Chemical identifiers
([O-])[O-].[N+](=O)([O-])[O-].[Hg+2] InChI identifier | InChI=1/Hg.2NO3/c;2*2-1(3)4/q+2;2*-1 EU number | 233-152-3 Gmelin number | 20106 RTECS number | OW8225000](../image_source/f88136de5b1ce2266822d0823171203f.png)
CAS number | 10045-94-0 PubChem CID number | 24864 SMILES identifier | [N+](=O)([O-])[O-].[N+](=O)([O-])[O-].[Hg+2] InChI identifier | InChI=1/Hg.2NO3/c;2*2-1(3)4/q+2;2*-1 EU number | 233-152-3 Gmelin number | 20106 RTECS number | OW8225000
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1
Toxicity properties
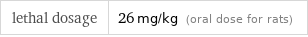
lethal dosage | 26 mg/kg (oral dose for rats)

probable lethal dose for man | 4 mL (milliliters) RTECS classes | other
Ion equivalents

Hg^(2+) (mercury(II) cation) | 1 (NO_3)^- (nitrate anion) | 2