Input interpretation
calcium fluoride
Chemical names and formulas

formula | CaF_2 name | calcium fluoride IUPAC name | calcium difluoride alternate names | acid-spar | calcium difluoride | irtran 3 | liparite | met-spar mass fractions | Ca (calcium) 51.3% | F (fluorine) 48.7%
Structure diagram
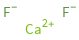
Structure diagram
Basic properties
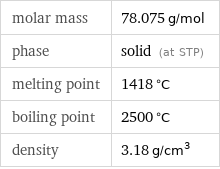
molar mass | 78.075 g/mol phase | solid (at STP) melting point | 1418 °C boiling point | 2500 °C density | 3.18 g/cm^3
Units

Solid properties (at STP)
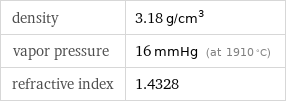
density | 3.18 g/cm^3 vapor pressure | 16 mmHg (at 1910 °C) refractive index | 1.4328
Units

Thermodynamic properties
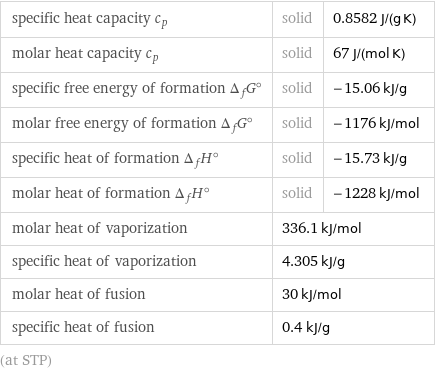
specific heat capacity c_p | solid | 0.8582 J/(g K) molar heat capacity c_p | solid | 67 J/(mol K) specific free energy of formation Δ_fG° | solid | -15.06 kJ/g molar free energy of formation Δ_fG° | solid | -1176 kJ/mol specific heat of formation Δ_fH° | solid | -15.73 kJ/g molar heat of formation Δ_fH° | solid | -1228 kJ/mol molar heat of vaporization | 336.1 kJ/mol | specific heat of vaporization | 4.305 kJ/g | molar heat of fusion | 30 kJ/mol | specific heat of fusion | 0.4 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7789-75-5 PubChem CID number | 24617 PubChem SID number | 24863582 SMILES identifier | [F-].[F-].[Ca+2] InChI identifier | InChI=1/Ca.2FH/h;2*1H/q+2;;/p-2/fCa.2F/h;2*1h/qm;2*-1 RTECS number | EW1760000 MDL number | MFCD00010907](../image_source/2d60f117b33ac9ab56164ef6f76e8d2b.png)
CAS number | 7789-75-5 PubChem CID number | 24617 PubChem SID number | 24863582 SMILES identifier | [F-].[F-].[Ca+2] InChI identifier | InChI=1/Ca.2FH/h;2*1H/q+2;;/p-2/fCa.2F/h;2*1h/qm;2*-1 RTECS number | EW1760000 MDL number | MFCD00010907
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties
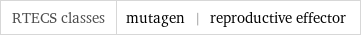
RTECS classes | mutagen | reproductive effector
Ion equivalents

Ca^(2+) (calcium cation) | 1 F^- (fluoride anion) | 2