Input interpretation

group 14 elements | first ionization energy
Summary
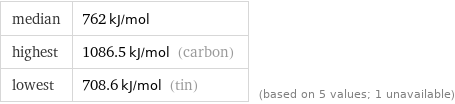
median | 762 kJ/mol highest | 1086.5 kJ/mol (carbon) lowest | 708.6 kJ/mol (tin) | (based on 5 values; 1 unavailable)
Entity with missing value
flerovium
Ranked values
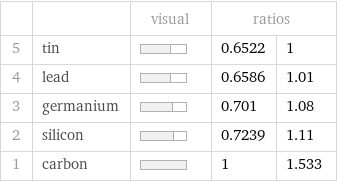
| | visual | ratios | 5 | tin | | 0.6522 | 1 4 | lead | | 0.6586 | 1.01 3 | germanium | | 0.701 | 1.08 2 | silicon | | 0.7239 | 1.11 1 | carbon | | 1 | 1.533
Plot
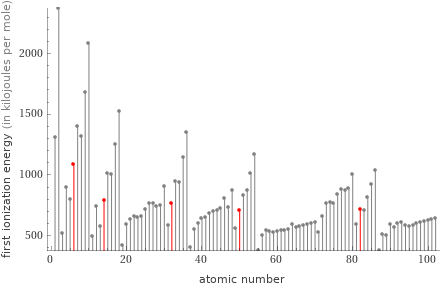
Plot
Periodic table location
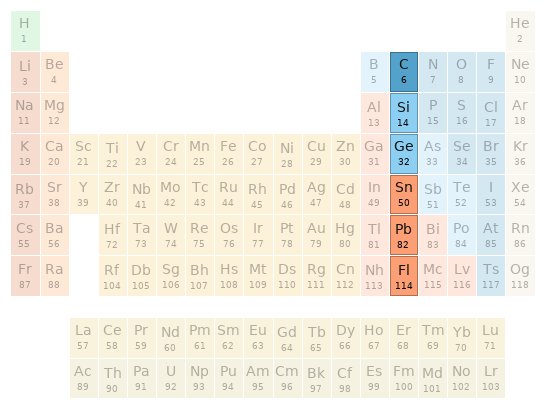
Periodic table location
First ionization energy rankings
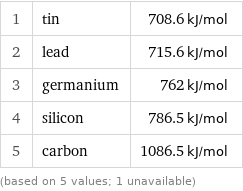
1 | tin | 708.6 kJ/mol 2 | lead | 715.6 kJ/mol 3 | germanium | 762 kJ/mol 4 | silicon | 786.5 kJ/mol 5 | carbon | 1086.5 kJ/mol (based on 5 values; 1 unavailable)
Unit conversions for median first ionization energy 762 kJ/mol

0.762 MJ/mol (megajoules per mole)
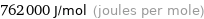
762000 J/mol (joules per mole)

182 kcal/mol (thermochemical kilocalories per mole)
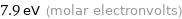
7.9 eV (molar electronvolts)
Comparisons for median first ionization energy 762 kJ/mol

≈ 0.32 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
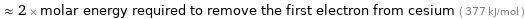
≈ 2 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )