Input interpretation
calcium hydroxide | pearl ash
Chemical names and formulas
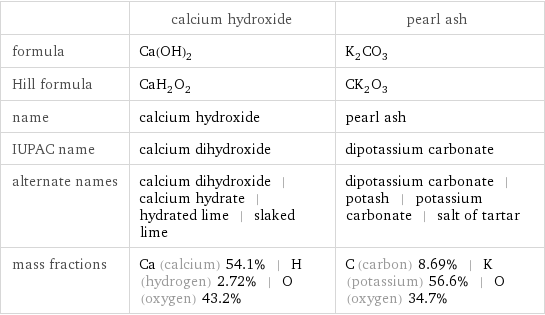
| calcium hydroxide | pearl ash formula | Ca(OH)_2 | K_2CO_3 Hill formula | CaH_2O_2 | CK_2O_3 name | calcium hydroxide | pearl ash IUPAC name | calcium dihydroxide | dipotassium carbonate alternate names | calcium dihydroxide | calcium hydrate | hydrated lime | slaked lime | dipotassium carbonate | potash | potassium carbonate | salt of tartar mass fractions | Ca (calcium) 54.1% | H (hydrogen) 2.72% | O (oxygen) 43.2% | C (carbon) 8.69% | K (potassium) 56.6% | O (oxygen) 34.7%
Structure diagrams
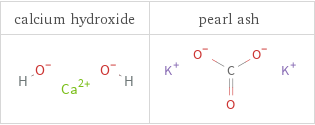
Structure diagrams
Basic properties
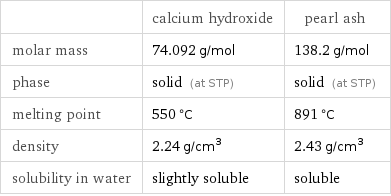
| calcium hydroxide | pearl ash molar mass | 74.092 g/mol | 138.2 g/mol phase | solid (at STP) | solid (at STP) melting point | 550 °C | 891 °C density | 2.24 g/cm^3 | 2.43 g/cm^3 solubility in water | slightly soluble | soluble
Units
