Input interpretation
lithium sulfate
Chemical names and formulas
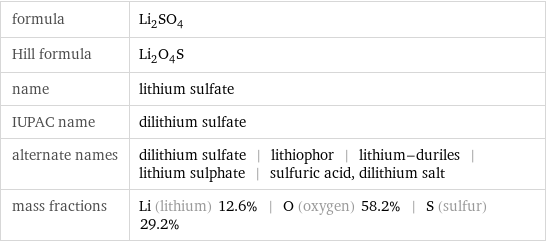
formula | Li_2SO_4 Hill formula | Li_2O_4S name | lithium sulfate IUPAC name | dilithium sulfate alternate names | dilithium sulfate | lithiophor | lithium-duriles | lithium sulphate | sulfuric acid, dilithium salt mass fractions | Li (lithium) 12.6% | O (oxygen) 58.2% | S (sulfur) 29.2%
Structure diagram
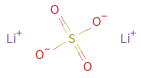
Structure diagram
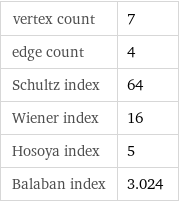
vertex count | 7 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
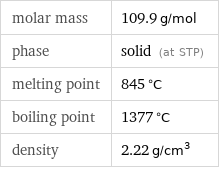
molar mass | 109.9 g/mol phase | solid (at STP) melting point | 845 °C boiling point | 1377 °C density | 2.22 g/cm^3
Units

Solid properties (at STP)

density | 2.22 g/cm^3
Units

Thermodynamic properties
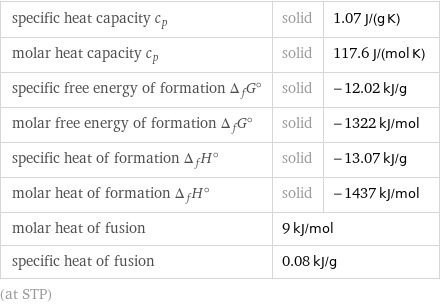
specific heat capacity c_p | solid | 1.07 J/(g K) molar heat capacity c_p | solid | 117.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -12.02 kJ/g molar free energy of formation Δ_fG° | solid | -1322 kJ/mol specific heat of formation Δ_fH° | solid | -13.07 kJ/g molar heat of formation Δ_fH° | solid | -1437 kJ/mol molar heat of fusion | 9 kJ/mol | specific heat of fusion | 0.08 kJ/g | (at STP)
Chemical identifiers
![CAS number | 10377-48-7 PubChem CID number | 66320 PubChem SID number | 24852189 SMILES identifier | [Li+].[Li+].[O-]S(=O)(=O)[O-] InChI identifier | InChI=1/2Li.H2O4S/c;;1-5(2, 3)4/h;;(H2, 1, 2, 3, 4)/q2*+1;/p-2/f2Li.O4S/q2m;-2 RTECS number | OJ6419000 MDL number | MFCD00011086](../image_source/dd966def2b5be205e69bd4d25a866288.png)
CAS number | 10377-48-7 PubChem CID number | 66320 PubChem SID number | 24852189 SMILES identifier | [Li+].[Li+].[O-]S(=O)(=O)[O-] InChI identifier | InChI=1/2Li.H2O4S/c;;1-5(2, 3)4/h;;(H2, 1, 2, 3, 4)/q2*+1;/p-2/f2Li.O4S/q2m;-2 RTECS number | OJ6419000 MDL number | MFCD00011086
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | drug | mutagen | human data
Ion equivalents

Li^+ (lithium cation) | 2 (SO_4)^(2-) (sulfate anion) | 1