Input interpretation
zinc chloride | magnesium chloride hexahydrate
Chemical names and formulas
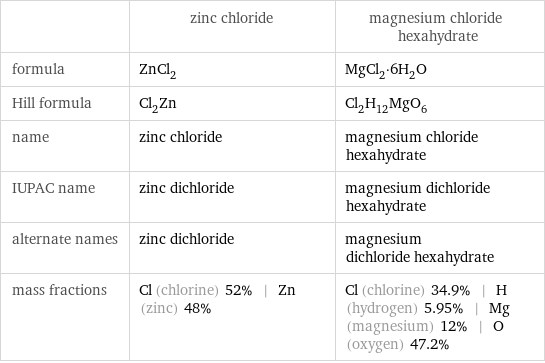
| zinc chloride | magnesium chloride hexahydrate formula | ZnCl_2 | MgCl_2·6H_2O Hill formula | Cl_2Zn | Cl_2H_12MgO_6 name | zinc chloride | magnesium chloride hexahydrate IUPAC name | zinc dichloride | magnesium dichloride hexahydrate alternate names | zinc dichloride | magnesium dichloride hexahydrate mass fractions | Cl (chlorine) 52% | Zn (zinc) 48% | Cl (chlorine) 34.9% | H (hydrogen) 5.95% | Mg (magnesium) 12% | O (oxygen) 47.2%
Structure diagrams
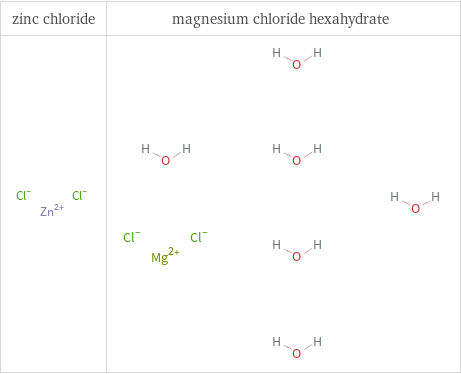
Structure diagrams
Basic properties
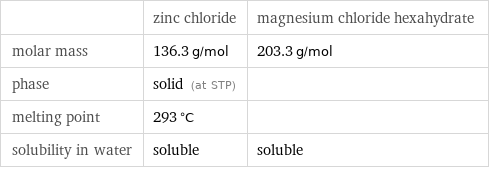
| zinc chloride | magnesium chloride hexahydrate molar mass | 136.3 g/mol | 203.3 g/mol phase | solid (at STP) | melting point | 293 °C | solubility in water | soluble | soluble
Units

Thermodynamic properties

| zinc chloride | magnesium chloride hexahydrate specific heat of fusion | 0.0756 kJ/g (kilojoules per gram) | 0.1668 kJ/g (kilojoules per gram)
Solid properties
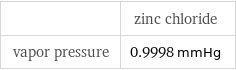
| zinc chloride vapor pressure | 0.9998 mmHg
Units
