Input interpretation

polar solvents | density
Summary
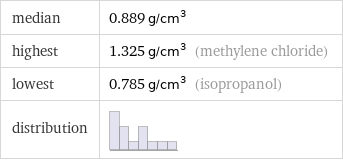
median | 0.889 g/cm^3 highest | 1.325 g/cm^3 (methylene chloride) lowest | 0.785 g/cm^3 (isopropanol) distribution |
Units

Distribution plots
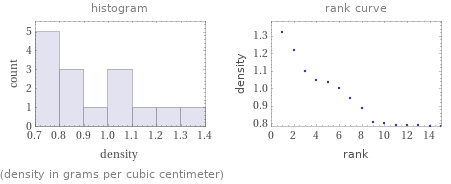
(density in grams per cubic centimeter)
Density rankings
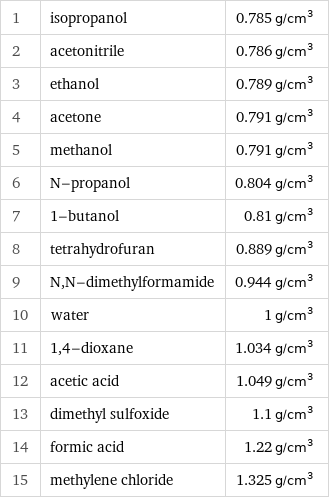
1 | isopropanol | 0.785 g/cm^3 2 | acetonitrile | 0.786 g/cm^3 3 | ethanol | 0.789 g/cm^3 4 | acetone | 0.791 g/cm^3 5 | methanol | 0.791 g/cm^3 6 | N-propanol | 0.804 g/cm^3 7 | 1-butanol | 0.81 g/cm^3 8 | tetrahydrofuran | 0.889 g/cm^3 9 | N, N-dimethylformamide | 0.944 g/cm^3 10 | water | 1 g/cm^3 11 | 1, 4-dioxane | 1.034 g/cm^3 12 | acetic acid | 1.049 g/cm^3 13 | dimethyl sulfoxide | 1.1 g/cm^3 14 | formic acid | 1.22 g/cm^3 15 | methylene chloride | 1.325 g/cm^3
Unit conversions for median density 0.889 g/cm^3
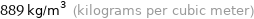
889 kg/m^3 (kilograms per cubic meter)
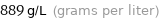
889 g/L (grams per liter)
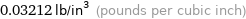
0.03212 lb/in^3 (pounds per cubic inch)
Comparisons for median density 0.889 g/cm^3
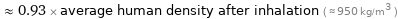
≈ 0.93 × average human density after inhalation ( ≈ 950 kg/m^3 )
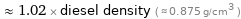
≈ 1.02 × diesel density ( ≈ 0.875 g/cm^3 )
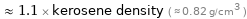
≈ 1.1 × kerosene density ( ≈ 0.82 g/cm^3 )