Input interpretation
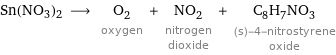
Sn(NO3)2 ⟶ O_2 oxygen + NO_2 nitrogen dioxide + C_8H_7NO_3 (s)-4-nitrostyrene oxide
Chemical names and formulas
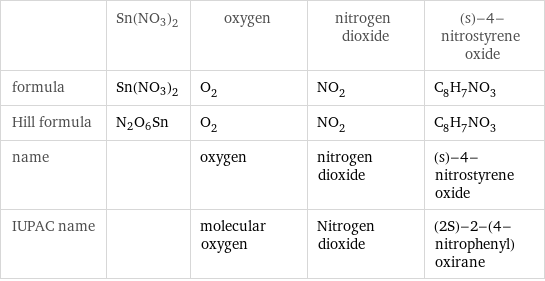
| Sn(NO3)2 | oxygen | nitrogen dioxide | (s)-4-nitrostyrene oxide formula | Sn(NO3)2 | O_2 | NO_2 | C_8H_7NO_3 Hill formula | N2O6Sn | O_2 | NO_2 | C_8H_7NO_3 name | | oxygen | nitrogen dioxide | (s)-4-nitrostyrene oxide IUPAC name | | molecular oxygen | Nitrogen dioxide | (2S)-2-(4-nitrophenyl)oxirane