Input interpretation

hydrogen fluoride | iodine
Chemical names and formulas
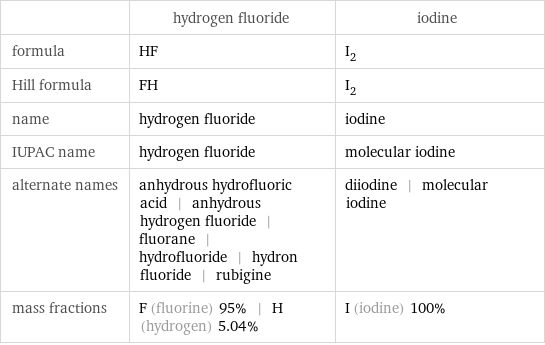
| hydrogen fluoride | iodine formula | HF | I_2 Hill formula | FH | I_2 name | hydrogen fluoride | iodine IUPAC name | hydrogen fluoride | molecular iodine alternate names | anhydrous hydrofluoric acid | anhydrous hydrogen fluoride | fluorane | hydrofluoride | hydron fluoride | rubigine | diiodine | molecular iodine mass fractions | F (fluorine) 95% | H (hydrogen) 5.04% | I (iodine) 100%
Structure diagrams

Structure diagrams
3D structure
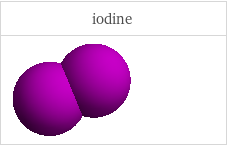
3D structure
Basic properties
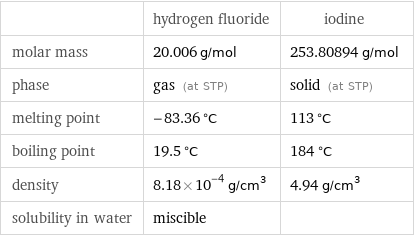
| hydrogen fluoride | iodine molar mass | 20.006 g/mol | 253.80894 g/mol phase | gas (at STP) | solid (at STP) melting point | -83.36 °C | 113 °C boiling point | 19.5 °C | 184 °C density | 8.18×10^-4 g/cm^3 | 4.94 g/cm^3 solubility in water | miscible |
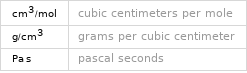
Units

Gas properties
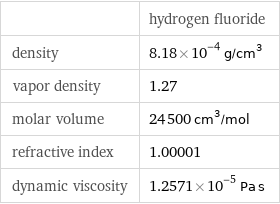
| hydrogen fluoride density | 8.18×10^-4 g/cm^3 vapor density | 1.27 molar volume | 24500 cm^3/mol refractive index | 1.00001 dynamic viscosity | 1.2571×10^-5 Pa s
Units
Thermodynamic properties
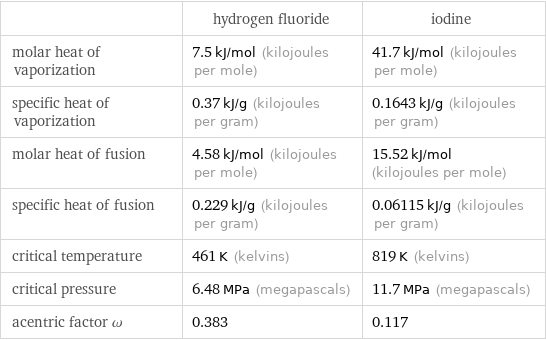
| hydrogen fluoride | iodine molar heat of vaporization | 7.5 kJ/mol (kilojoules per mole) | 41.7 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.37 kJ/g (kilojoules per gram) | 0.1643 kJ/g (kilojoules per gram) molar heat of fusion | 4.58 kJ/mol (kilojoules per mole) | 15.52 kJ/mol (kilojoules per mole) specific heat of fusion | 0.229 kJ/g (kilojoules per gram) | 0.06115 kJ/g (kilojoules per gram) critical temperature | 461 K (kelvins) | 819 K (kelvins) critical pressure | 6.48 MPa (megapascals) | 11.7 MPa (megapascals) acentric factor ω | 0.383 | 0.117
Solid properties
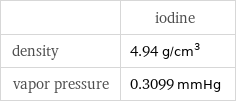
| iodine density | 4.94 g/cm^3 vapor pressure | 0.3099 mmHg
Units

Chemical identifiers
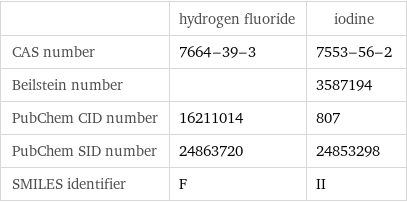
| hydrogen fluoride | iodine CAS number | 7664-39-3 | 7553-56-2 Beilstein number | | 3587194 PubChem CID number | 16211014 | 807 PubChem SID number | 24863720 | 24853298 SMILES identifier | F | II
NFPA label

NFPA label