Input interpretation
iodine (chemical element) | astatine (chemical element) | molybdenum (chemical element) | neptunium (chemical element)
Periodic table location
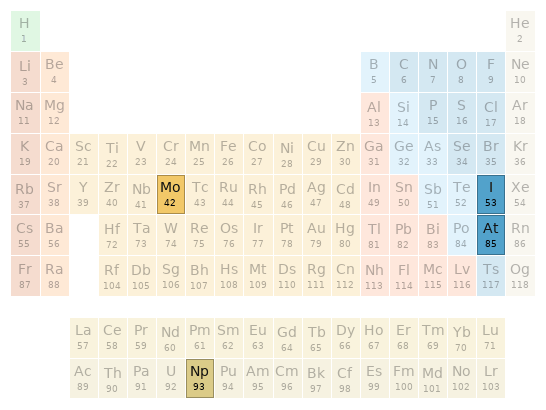
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration iodine | I | 53 | [Kr]5s^24d^105p^5 astatine | At | 85 | [Xe]6s^24f^145d^106p^5 molybdenum | Mo | 42 | [Kr]5s^14d^5 neptunium | Np | 93 | [Rn]7s^25f^46d^1 | Aufbau diagram | block | group iodine | 5p 4d 5s | p | 17 astatine | 6p 5d 4f 6s | p | 17 molybdenum | 4d 5s | d | 6 neptunium | 6d 5f 7s | f | | period | atomic mass | half-life iodine | 5 | 126.90447 u | (stable) astatine | 6 | 210 u | 8.1 h molybdenum | 5 | 95.95 u | (stable) neptunium | 7 | 237 u | 2.145 Myr](../image_source/c82b435454f0822102fdddb468e3a716.png)
| atomic symbol | atomic number | short electronic configuration iodine | I | 53 | [Kr]5s^24d^105p^5 astatine | At | 85 | [Xe]6s^24f^145d^106p^5 molybdenum | Mo | 42 | [Kr]5s^14d^5 neptunium | Np | 93 | [Rn]7s^25f^46d^1 | Aufbau diagram | block | group iodine | 5p 4d 5s | p | 17 astatine | 6p 5d 4f 6s | p | 17 molybdenum | 4d 5s | d | 6 neptunium | 6d 5f 7s | f | | period | atomic mass | half-life iodine | 5 | 126.90447 u | (stable) astatine | 6 | 210 u | 8.1 h molybdenum | 5 | 95.95 u | (stable) neptunium | 7 | 237 u | 2.145 Myr
Thermodynamic properties
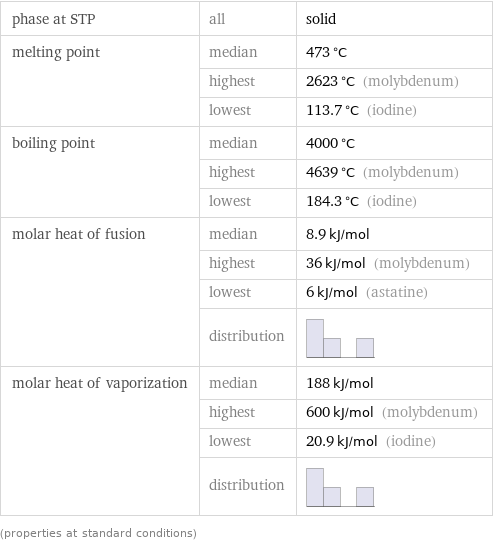
phase at STP | all | solid melting point | median | 473 °C | highest | 2623 °C (molybdenum) | lowest | 113.7 °C (iodine) boiling point | median | 4000 °C | highest | 4639 °C (molybdenum) | lowest | 184.3 °C (iodine) molar heat of fusion | median | 8.9 kJ/mol | highest | 36 kJ/mol (molybdenum) | lowest | 6 kJ/mol (astatine) | distribution | molar heat of vaporization | median | 188 kJ/mol | highest | 600 kJ/mol (molybdenum) | lowest | 20.9 kJ/mol (iodine) | distribution | (properties at standard conditions)
Material properties
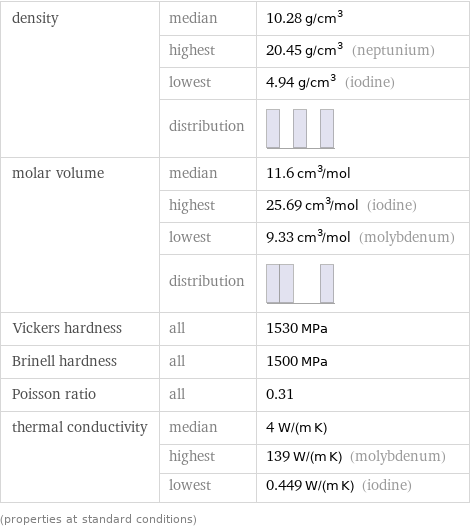
density | median | 10.28 g/cm^3 | highest | 20.45 g/cm^3 (neptunium) | lowest | 4.94 g/cm^3 (iodine) | distribution | molar volume | median | 11.6 cm^3/mol | highest | 25.69 cm^3/mol (iodine) | lowest | 9.33 cm^3/mol (molybdenum) | distribution | Vickers hardness | all | 1530 MPa Brinell hardness | all | 1500 MPa Poisson ratio | all | 0.31 thermal conductivity | median | 4 W/(m K) | highest | 139 W/(m K) (molybdenum) | lowest | 0.449 W/(m K) (iodine) (properties at standard conditions)
Electromagnetic properties
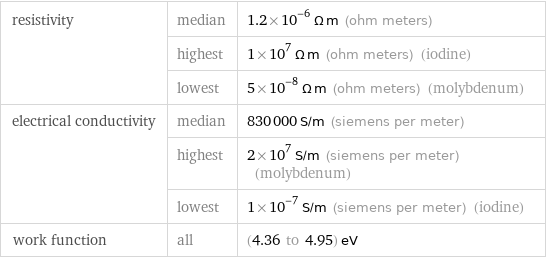
resistivity | median | 1.2×10^-6 Ω m (ohm meters) | highest | 1×10^7 Ω m (ohm meters) (iodine) | lowest | 5×10^-8 Ω m (ohm meters) (molybdenum) electrical conductivity | median | 830000 S/m (siemens per meter) | highest | 2×10^7 S/m (siemens per meter) (molybdenum) | lowest | 1×10^-7 S/m (siemens per meter) (iodine) work function | all | (4.36 to 4.95) eV
Reactivity
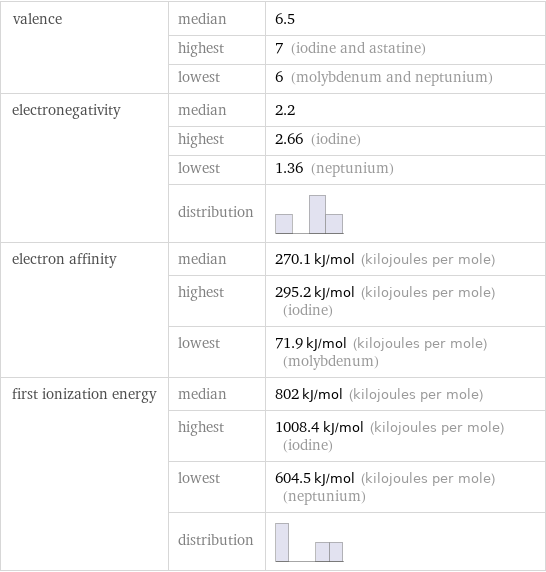
valence | median | 6.5 | highest | 7 (iodine and astatine) | lowest | 6 (molybdenum and neptunium) electronegativity | median | 2.2 | highest | 2.66 (iodine) | lowest | 1.36 (neptunium) | distribution | electron affinity | median | 270.1 kJ/mol (kilojoules per mole) | highest | 295.2 kJ/mol (kilojoules per mole) (iodine) | lowest | 71.9 kJ/mol (kilojoules per mole) (molybdenum) first ionization energy | median | 802 kJ/mol (kilojoules per mole) | highest | 1008.4 kJ/mol (kilojoules per mole) (iodine) | lowest | 604.5 kJ/mol (kilojoules per mole) (neptunium) | distribution |
Atomic properties
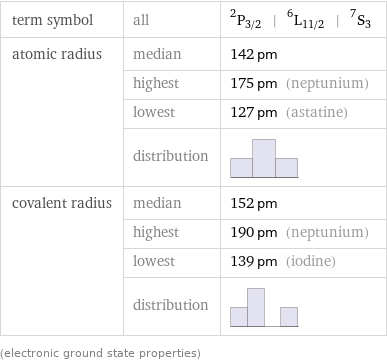
term symbol | all | ^2P_(3/2) | ^6L_(11/2) | ^7S_3 atomic radius | median | 142 pm | highest | 175 pm (neptunium) | lowest | 127 pm (astatine) | distribution | covalent radius | median | 152 pm | highest | 190 pm (neptunium) | lowest | 139 pm (iodine) | distribution | (electronic ground state properties)
Abundances
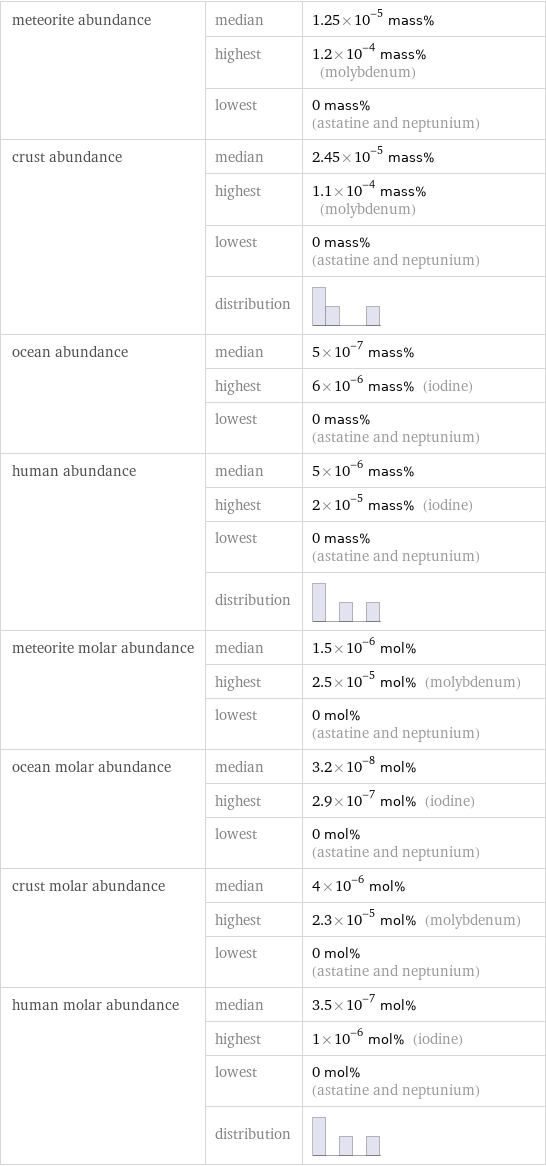
meteorite abundance | median | 1.25×10^-5 mass% | highest | 1.2×10^-4 mass% (molybdenum) | lowest | 0 mass% (astatine and neptunium) crust abundance | median | 2.45×10^-5 mass% | highest | 1.1×10^-4 mass% (molybdenum) | lowest | 0 mass% (astatine and neptunium) | distribution | ocean abundance | median | 5×10^-7 mass% | highest | 6×10^-6 mass% (iodine) | lowest | 0 mass% (astatine and neptunium) human abundance | median | 5×10^-6 mass% | highest | 2×10^-5 mass% (iodine) | lowest | 0 mass% (astatine and neptunium) | distribution | meteorite molar abundance | median | 1.5×10^-6 mol% | highest | 2.5×10^-5 mol% (molybdenum) | lowest | 0 mol% (astatine and neptunium) ocean molar abundance | median | 3.2×10^-8 mol% | highest | 2.9×10^-7 mol% (iodine) | lowest | 0 mol% (astatine and neptunium) crust molar abundance | median | 4×10^-6 mol% | highest | 2.3×10^-5 mol% (molybdenum) | lowest | 0 mol% (astatine and neptunium) human molar abundance | median | 3.5×10^-7 mol% | highest | 1×10^-6 mol% (iodine) | lowest | 0 mol% (astatine and neptunium) | distribution |