Input interpretation
actinium (chemical element) | francium (chemical element)
Periodic table location
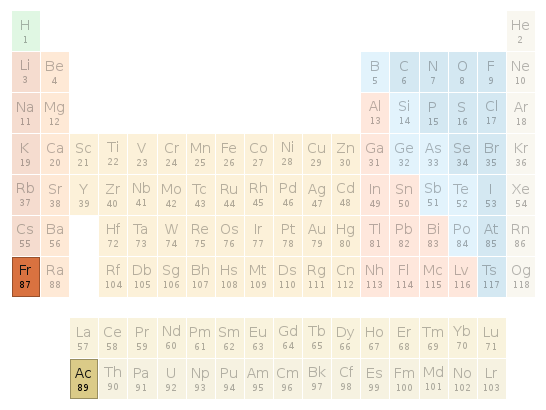
Periodic table location
Images
Images
Basic elemental properties
![| actinium | francium atomic symbol | Ac | Fr atomic number | 89 | 87 short electronic configuration | [Rn]7s^26d^1 | [Rn]7s^1 Aufbau diagram | 6d 7s | 7s block | f | s group | | 1 period | 7 | 7 atomic mass | 227 u | 223 u half-life | 21.787 yr | 22 min](../image_source/d69ac3cdb2be757f61ff65a3950aad57.png)
| actinium | francium atomic symbol | Ac | Fr atomic number | 89 | 87 short electronic configuration | [Rn]7s^26d^1 | [Rn]7s^1 Aufbau diagram | 6d 7s | 7s block | f | s group | | 1 period | 7 | 7 atomic mass | 227 u | 223 u half-life | 21.787 yr | 22 min
Thermodynamic properties
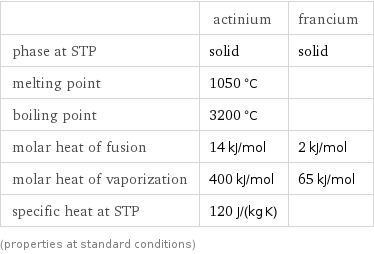
| actinium | francium phase at STP | solid | solid melting point | 1050 °C | boiling point | 3200 °C | molar heat of fusion | 14 kJ/mol | 2 kJ/mol molar heat of vaporization | 400 kJ/mol | 65 kJ/mol specific heat at STP | 120 J/(kg K) | (properties at standard conditions)
Material properties
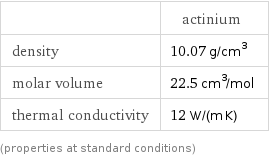
| actinium density | 10.07 g/cm^3 molar volume | 22.5 cm^3/mol thermal conductivity | 12 W/(m K) (properties at standard conditions)
Electromagnetic properties

| actinium | francium color | (silver) | (silver)
Reactivity
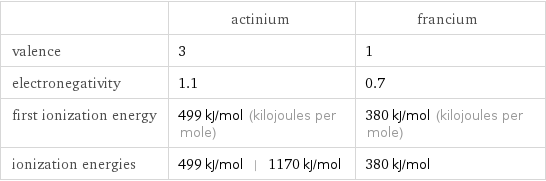
| actinium | francium valence | 3 | 1 electronegativity | 1.1 | 0.7 first ionization energy | 499 kJ/mol (kilojoules per mole) | 380 kJ/mol (kilojoules per mole) ionization energies | 499 kJ/mol | 1170 kJ/mol | 380 kJ/mol
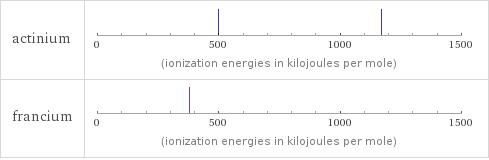
Reactivity
Atomic properties
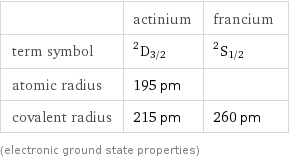
| actinium | francium term symbol | ^2D_(3/2) | ^2S_(1/2) atomic radius | 195 pm | covalent radius | 215 pm | 260 pm (electronic ground state properties)
Abundances
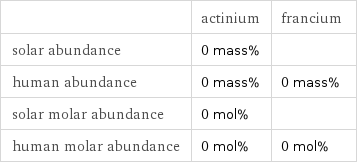
| actinium | francium solar abundance | 0 mass% | human abundance | 0 mass% | 0 mass% solar molar abundance | 0 mol% | human molar abundance | 0 mol% | 0 mol%
Nuclear properties

| actinium | francium half-life | 21.787 yr | 22 min lifetime | 31.431 yr | 32 min specific radioactivity | 2.676 TBq/g | 1.421×10^6 TBq/g decay mode | beta decay | beta decay nuclear spin | Ac-206: 3^+ | Ac-207: 9/2^- | Ac-208: 3^+ | Ac-209: 9/2^- | Ac-210: 7^+ | Ac-211: 9/2^- | Ac-212: 6^+ | Ac-213: 9/2^- | Ac-214: 5^+ | Ac-215: 9/2^- | Ac-216: 1^- | Ac-217: 9/2^- | Ac-218: 1^- | Ac-219: 9/2^- | Ac-220: 3^- | Ac-221: 3/2^- | Ac-222: 1^- | Ac-223: 5/2^- | Ac-224: 0^- | Ac-225: 3/2^- | Ac-226: 1^- | Ac-227: 3/2^- | Ac-228: 3^+ | Ac-229: 3/2^+ | Ac-230: 1^+ | Ac-231: 1/2^+ | Ac-232: 1^+ | Ac-233: 1/2^+ | Ac-235: 1/2^+ | Fr-199: 1/2^+ | Fr-200: 3^+ | Fr-201: 9/2^- | Fr-202: 3^+ | Fr-203: 9/2^- | Fr-204: 3^+ | Fr-205: 9/2^- | Fr-207: 9/2^- | Fr-208: 7^+ | Fr-209: 9/2^- | Fr-210: 6^+ | Fr-211: 9/2^- | Fr-212: 5^+ | Fr-213: 9/2^- | Fr-214: 1^- | Fr-215: 9/2^- | Fr-216: 1^- | Fr-217: 9/2^- | Fr-218: 1^- | Fr-219: 9/2^- | Fr-220: 1^+ | Fr-221: 5/2^- | Fr-222: 2^- | Fr-223: 3/2^- | Fr-224: 1^- | Fr-225: 3/2^- | Fr-226: 1^- | Fr-227: 1/2^+ | Fr-228: 2^- | Fr-229: 1/2^+ | Fr-231: 1/2^+ | Fr-232: 5^? unstable isotopes | Ac-227 (21.772 yr) | Ac-225 (10 days) | Ac-226 (29.36 h) | Ac-228 (6.14 h) | Ac-224 (170 min) | Ac-229 (62.67 min) | Ac-231 (7.5 min) | Ac-233 (145 s) | Ac-223 (126 s) | Ac-230 (122 s) | Ac-232 (119 s) | Ac-236 (100 s) | Ac-235 (60 s) | Ac-234 (44 s) | Ac-214 (8.2 s) | Ac-222 (5 s) | Ac-212 (930 ms) | Ac-213 (738 ms) | Ac-210 (350 ms) | Ac-211 (210 ms) | Ac-215 (170 ms) | Ac-209 (100 ms) | Ac-208 (95 ms) | Ac-221 (52 ms) | Ac-207 (27 ms) | Ac-220 (26.36 ms) | Ac-206 (22 ms) | Ac-216 (440 µs) | Ac-219 (11.8 µs) | Ac-218 (1.08 µs) | Ac-217 (69 ns) | Fr-223 (22 min) | Fr-212 (20 min) | Fr-222 (14.2 min) | Fr-221 (4.83 min) | Fr-225 (3.95 min) | Fr-224 (200 s) | Fr-210 (191 s) | Fr-211 (186 s) | Fr-227 (148 s) | Fr-208 (59.1 s) | Fr-229 (50.2 s) | Fr-209 (50 s) | Fr-226 (49 s) | Fr-228 (38 s) | Fr-213 (34.82 s) | Fr-220 (27.4 s) | Fr-230 (19.1 s) | Fr-231 (17.6 s) | Fr-206 (16 s) | Fr-207 (14.8 s) | Fr-232 (5.5 s) | Fr-205 (3.8 s) | Fr-204 (1.7 s) | Fr-203 (550 ms) | Fr-202 (300 ms) | Fr-201 (61 ms) | Fr-200 (49 ms) | Fr-219 (20 ms) | Fr-199 (12 ms) | Fr-214 (5 ms) | Fr-218 (1 ms) | Fr-217 (19 µs) | Fr-216 (700 ns) | Fr-215 (86 ns) neutron cross-section | 880 b | neutron mass absorption | 0.079 m^2/kg |