Input interpretation

indium (chemical element) | nihonium (chemical element) | aluminum (chemical element) | gallium (chemical element) | thallium (chemical element) | livermorium (chemical element) | tin (chemical element) | lead (chemical element)
Periodic table location
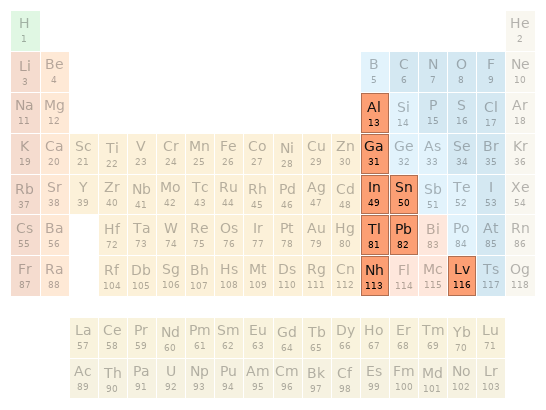
Periodic table location
Images
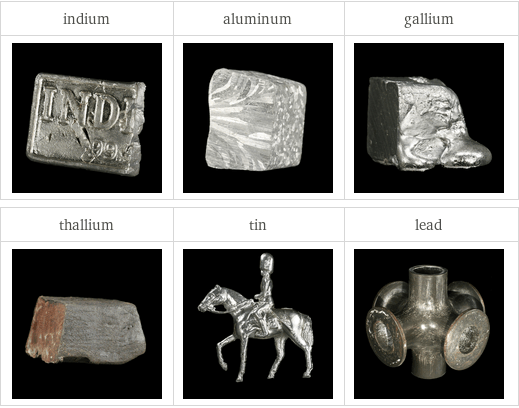
Images
Basic elemental properties
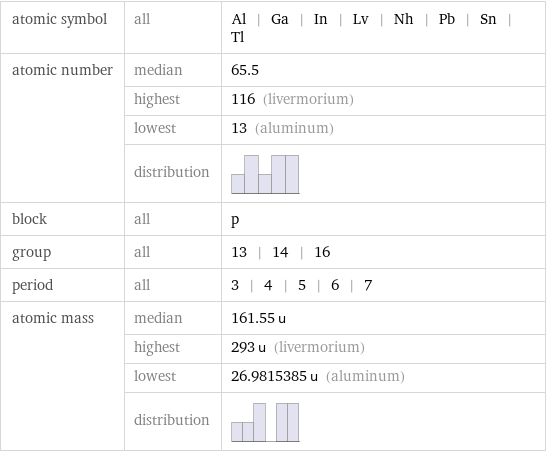
atomic symbol | all | Al | Ga | In | Lv | Nh | Pb | Sn | Tl atomic number | median | 65.5 | highest | 116 (livermorium) | lowest | 13 (aluminum) | distribution | block | all | p group | all | 13 | 14 | 16 period | all | 3 | 4 | 5 | 6 | 7 atomic mass | median | 161.55 u | highest | 293 u (livermorium) | lowest | 26.9815385 u (aluminum) | distribution |
Thermodynamic properties
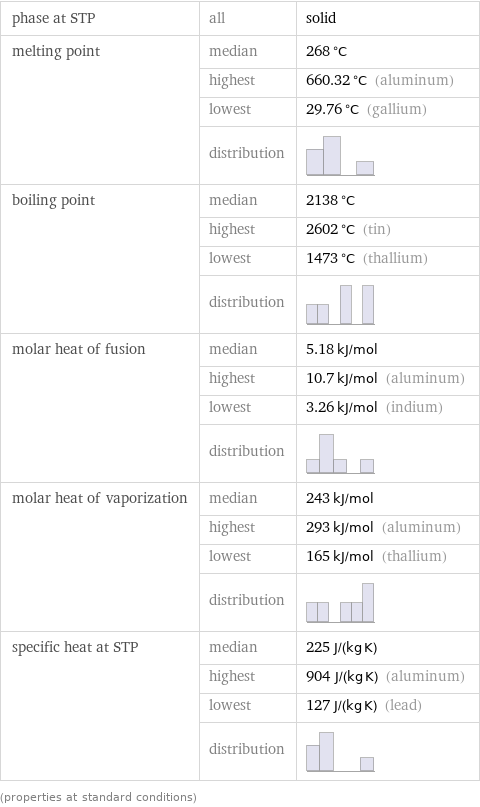
phase at STP | all | solid melting point | median | 268 °C | highest | 660.32 °C (aluminum) | lowest | 29.76 °C (gallium) | distribution | boiling point | median | 2138 °C | highest | 2602 °C (tin) | lowest | 1473 °C (thallium) | distribution | molar heat of fusion | median | 5.18 kJ/mol | highest | 10.7 kJ/mol (aluminum) | lowest | 3.26 kJ/mol (indium) | distribution | molar heat of vaporization | median | 243 kJ/mol | highest | 293 kJ/mol (aluminum) | lowest | 165 kJ/mol (thallium) | distribution | specific heat at STP | median | 225 J/(kg K) | highest | 904 J/(kg K) (aluminum) | lowest | 127 J/(kg K) (lead) | distribution | (properties at standard conditions)
Material properties
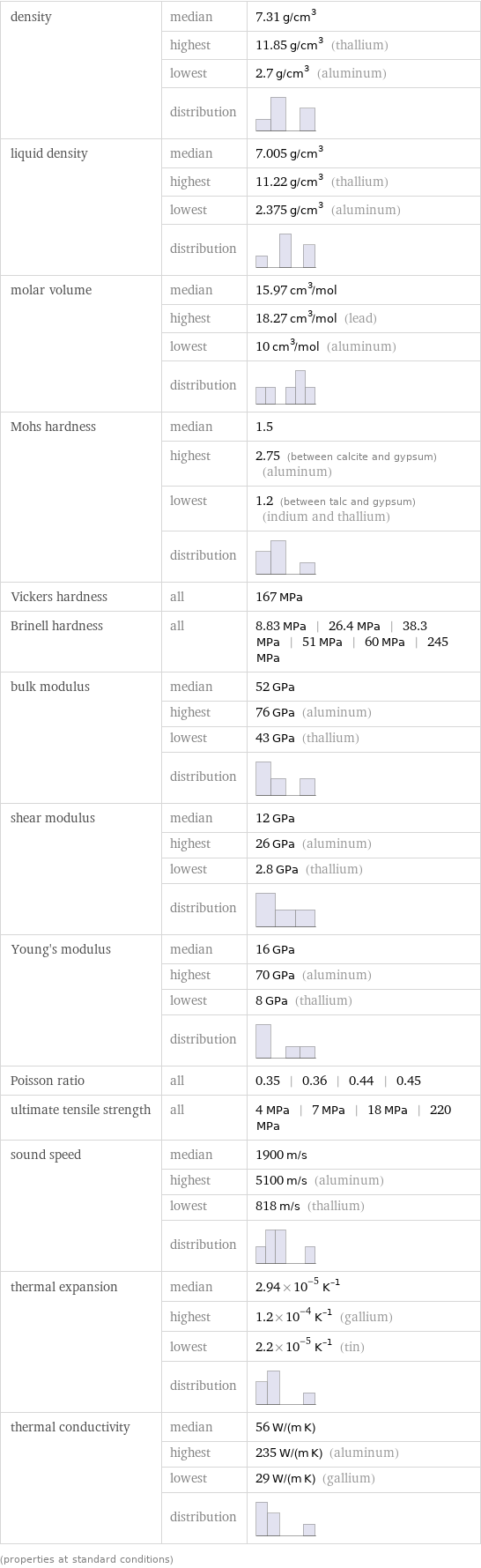
density | median | 7.31 g/cm^3 | highest | 11.85 g/cm^3 (thallium) | lowest | 2.7 g/cm^3 (aluminum) | distribution | liquid density | median | 7.005 g/cm^3 | highest | 11.22 g/cm^3 (thallium) | lowest | 2.375 g/cm^3 (aluminum) | distribution | molar volume | median | 15.97 cm^3/mol | highest | 18.27 cm^3/mol (lead) | lowest | 10 cm^3/mol (aluminum) | distribution | Mohs hardness | median | 1.5 | highest | 2.75 (between calcite and gypsum) (aluminum) | lowest | 1.2 (between talc and gypsum) (indium and thallium) | distribution | Vickers hardness | all | 167 MPa Brinell hardness | all | 8.83 MPa | 26.4 MPa | 38.3 MPa | 51 MPa | 60 MPa | 245 MPa bulk modulus | median | 52 GPa | highest | 76 GPa (aluminum) | lowest | 43 GPa (thallium) | distribution | shear modulus | median | 12 GPa | highest | 26 GPa (aluminum) | lowest | 2.8 GPa (thallium) | distribution | Young's modulus | median | 16 GPa | highest | 70 GPa (aluminum) | lowest | 8 GPa (thallium) | distribution | Poisson ratio | all | 0.35 | 0.36 | 0.44 | 0.45 ultimate tensile strength | all | 4 MPa | 7 MPa | 18 MPa | 220 MPa sound speed | median | 1900 m/s | highest | 5100 m/s (aluminum) | lowest | 818 m/s (thallium) | distribution | thermal expansion | median | 2.94×10^-5 K^(-1) | highest | 1.2×10^-4 K^(-1) (gallium) | lowest | 2.2×10^-5 K^(-1) (tin) | distribution | thermal conductivity | median | 56 W/(m K) | highest | 235 W/(m K) (aluminum) | lowest | 29 W/(m K) (gallium) | distribution | (properties at standard conditions)
Electromagnetic properties
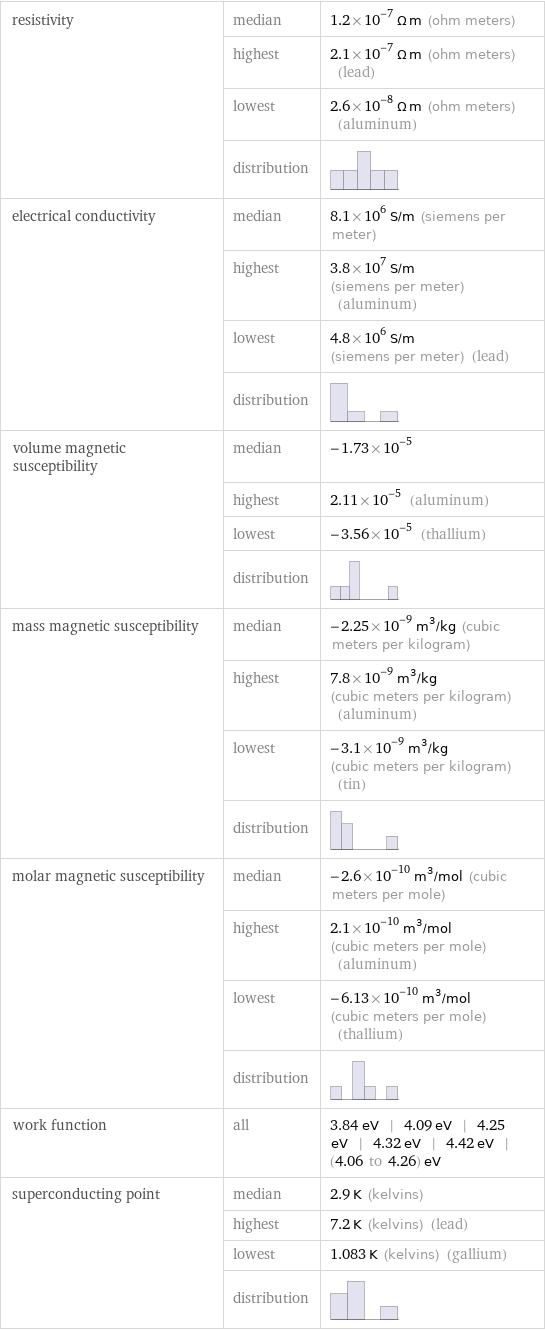
resistivity | median | 1.2×10^-7 Ω m (ohm meters) | highest | 2.1×10^-7 Ω m (ohm meters) (lead) | lowest | 2.6×10^-8 Ω m (ohm meters) (aluminum) | distribution | electrical conductivity | median | 8.1×10^6 S/m (siemens per meter) | highest | 3.8×10^7 S/m (siemens per meter) (aluminum) | lowest | 4.8×10^6 S/m (siemens per meter) (lead) | distribution | volume magnetic susceptibility | median | -1.73×10^-5 | highest | 2.11×10^-5 (aluminum) | lowest | -3.56×10^-5 (thallium) | distribution | mass magnetic susceptibility | median | -2.25×10^-9 m^3/kg (cubic meters per kilogram) | highest | 7.8×10^-9 m^3/kg (cubic meters per kilogram) (aluminum) | lowest | -3.1×10^-9 m^3/kg (cubic meters per kilogram) (tin) | distribution | molar magnetic susceptibility | median | -2.6×10^-10 m^3/mol (cubic meters per mole) | highest | 2.1×10^-10 m^3/mol (cubic meters per mole) (aluminum) | lowest | -6.13×10^-10 m^3/mol (cubic meters per mole) (thallium) | distribution | work function | all | 3.84 eV | 4.09 eV | 4.25 eV | 4.32 eV | 4.42 eV | (4.06 to 4.26) eV superconducting point | median | 2.9 K (kelvins) | highest | 7.2 K (kelvins) (lead) | lowest | 1.083 K (kelvins) (gallium) | distribution |
Reactivity
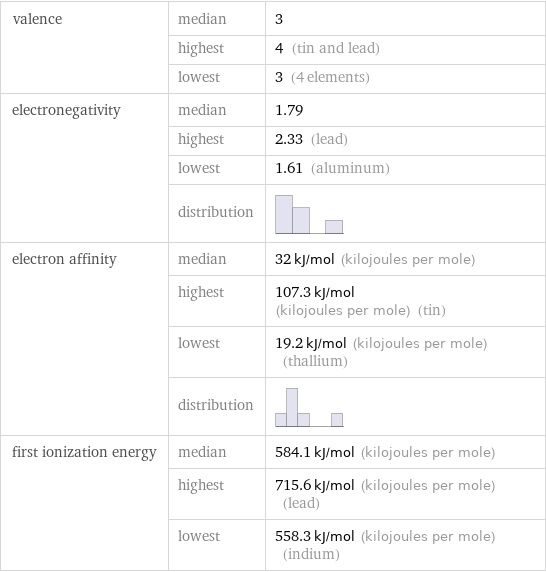
valence | median | 3 | highest | 4 (tin and lead) | lowest | 3 (4 elements) electronegativity | median | 1.79 | highest | 2.33 (lead) | lowest | 1.61 (aluminum) | distribution | electron affinity | median | 32 kJ/mol (kilojoules per mole) | highest | 107.3 kJ/mol (kilojoules per mole) (tin) | lowest | 19.2 kJ/mol (kilojoules per mole) (thallium) | distribution | first ionization energy | median | 584.1 kJ/mol (kilojoules per mole) | highest | 715.6 kJ/mol (kilojoules per mole) (lead) | lowest | 558.3 kJ/mol (kilojoules per mole) (indium)
Atomic properties
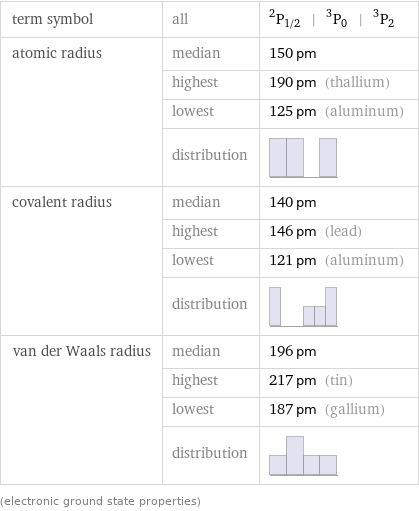
term symbol | all | ^2P_(1/2) | ^3P_0 | ^3P_2 atomic radius | median | 150 pm | highest | 190 pm (thallium) | lowest | 125 pm (aluminum) | distribution | covalent radius | median | 140 pm | highest | 146 pm (lead) | lowest | 121 pm (aluminum) | distribution | van der Waals radius | median | 196 pm | highest | 217 pm (tin) | lowest | 187 pm (gallium) | distribution | (electronic ground state properties)