Input interpretation
white tin
Chemical names and formulas
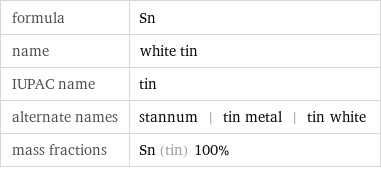
formula | Sn name | white tin IUPAC name | tin alternate names | stannum | tin metal | tin white mass fractions | Sn (tin) 100%
Structure diagram
Structure diagram
Basic properties
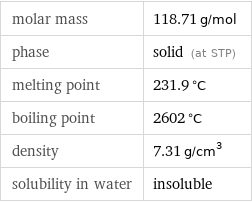
molar mass | 118.71 g/mol phase | solid (at STP) melting point | 231.9 °C boiling point | 2602 °C density | 7.31 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 7.31 g/cm^3 vapor pressure | 6×10^-7 mmHg (at 800 °C)
Units

Thermodynamic properties
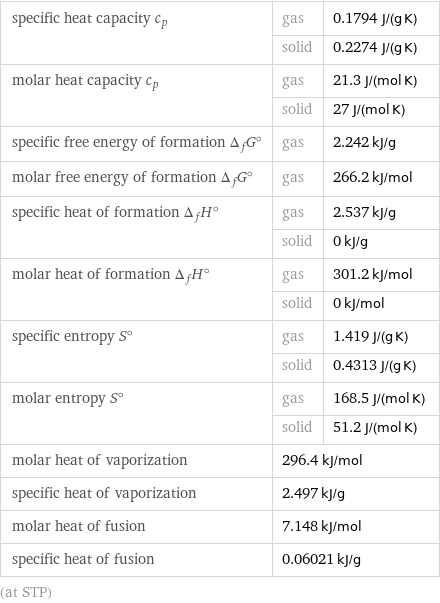
specific heat capacity c_p | gas | 0.1794 J/(g K) | solid | 0.2274 J/(g K) molar heat capacity c_p | gas | 21.3 J/(mol K) | solid | 27 J/(mol K) specific free energy of formation Δ_fG° | gas | 2.242 kJ/g molar free energy of formation Δ_fG° | gas | 266.2 kJ/mol specific heat of formation Δ_fH° | gas | 2.537 kJ/g | solid | 0 kJ/g molar heat of formation Δ_fH° | gas | 301.2 kJ/mol | solid | 0 kJ/mol specific entropy S° | gas | 1.419 J/(g K) | solid | 0.4313 J/(g K) molar entropy S° | gas | 168.5 J/(mol K) | solid | 51.2 J/(mol K) molar heat of vaporization | 296.4 kJ/mol | specific heat of vaporization | 2.497 kJ/g | molar heat of fusion | 7.148 kJ/mol | specific heat of fusion | 0.06021 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7440-31-5 PubChem CID number | 5352426 PubChem SID number | 24852287 SMILES identifier | [Sn] InChI identifier | InChI=1/Sn RTECS number | XP7320000 MDL number | MFCD00133862](../image_source/6dcb07a3a7fc870e0dffcf5246c8739c.png)
CAS number | 7440-31-5 PubChem CID number | 5352426 PubChem SID number | 24852287 SMILES identifier | [Sn] InChI identifier | InChI=1/Sn RTECS number | XP7320000 MDL number | MFCD00133862
NFPA label

NFPA label

NFPA health rating | 1 NFPA fire rating | 3 NFPA reactivity rating | 3
Safety properties

autoignition point | 630 °C
Toxicity properties

odor | odorless

RTECS classes | tumorigen