Input interpretation

weak electrolytes | relative molecular mass
Summary
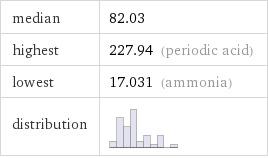
median | 82.03 highest | 227.94 (periodic acid) lowest | 17.031 (ammonia) distribution |
Distribution plots
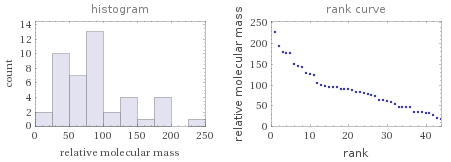
Relative molecular mass rankings
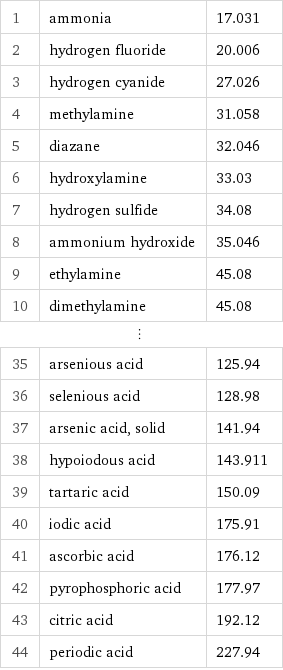
1 | ammonia | 17.031 2 | hydrogen fluoride | 20.006 3 | hydrogen cyanide | 27.026 4 | methylamine | 31.058 5 | diazane | 32.046 6 | hydroxylamine | 33.03 7 | hydrogen sulfide | 34.08 8 | ammonium hydroxide | 35.046 9 | ethylamine | 45.08 10 | dimethylamine | 45.08 ⋮ | | 35 | arsenious acid | 125.94 36 | selenious acid | 128.98 37 | arsenic acid, solid | 141.94 38 | hypoiodous acid | 143.911 39 | tartaric acid | 150.09 40 | iodic acid | 175.91 41 | ascorbic acid | 176.12 42 | pyrophosphoric acid | 177.97 43 | citric acid | 192.12 44 | periodic acid | 227.94
Comparisons for median relative molecular mass 82.03
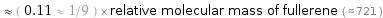
≈ ( 0.11 ≈ 1/9 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.42 × relative molecular mass of caffeine ( ≈ 194 )
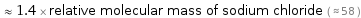
≈ 1.4 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
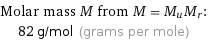
Molar mass M from M = M_uM_r: | 82 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 1.4×10^-22 grams | 1.4×10^-25 kg (kilograms)