Input interpretation
lithium iodide | sodium sulfide
Chemical names and formulas
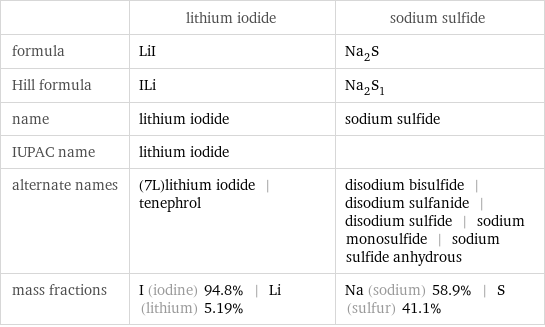
| lithium iodide | sodium sulfide formula | LiI | Na_2S Hill formula | ILi | Na_2S_1 name | lithium iodide | sodium sulfide IUPAC name | lithium iodide | alternate names | (7L)lithium iodide | tenephrol | disodium bisulfide | disodium sulfanide | disodium sulfide | sodium monosulfide | sodium sulfide anhydrous mass fractions | I (iodine) 94.8% | Li (lithium) 5.19% | Na (sodium) 58.9% | S (sulfur) 41.1%
Structure diagrams
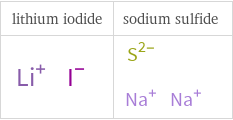
Structure diagrams
Basic properties
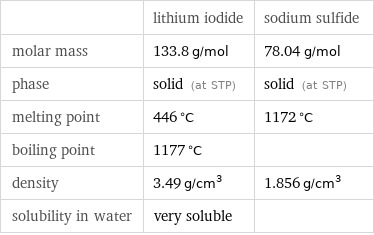
| lithium iodide | sodium sulfide molar mass | 133.8 g/mol | 78.04 g/mol phase | solid (at STP) | solid (at STP) melting point | 446 °C | 1172 °C boiling point | 1177 °C | density | 3.49 g/cm^3 | 1.856 g/cm^3 solubility in water | very soluble |
Units

Thermodynamic properties
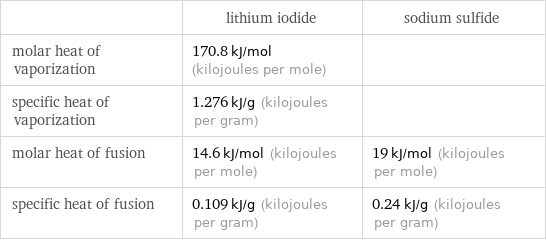
| lithium iodide | sodium sulfide molar heat of vaporization | 170.8 kJ/mol (kilojoules per mole) | specific heat of vaporization | 1.276 kJ/g (kilojoules per gram) | molar heat of fusion | 14.6 kJ/mol (kilojoules per mole) | 19 kJ/mol (kilojoules per mole) specific heat of fusion | 0.109 kJ/g (kilojoules per gram) | 0.24 kJ/g (kilojoules per gram)