Input interpretation

monoprotic acids | molar volume
Summary
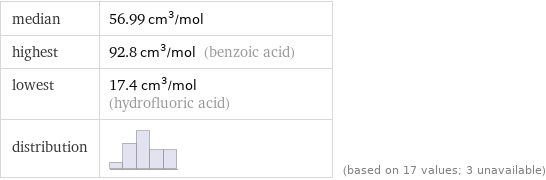
median | 56.99 cm^3/mol highest | 92.8 cm^3/mol (benzoic acid) lowest | 17.4 cm^3/mol (hydrofluoric acid) distribution | | (based on 17 values; 3 unavailable)
Entities with missing values
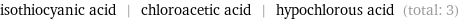
isothiocyanic acid | chloroacetic acid | hypochlorous acid (total: 3)
Distribution plots
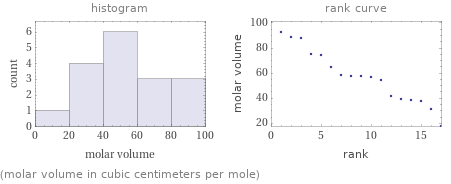
(molar volume in cubic centimeters per mole)
Molar volume rankings
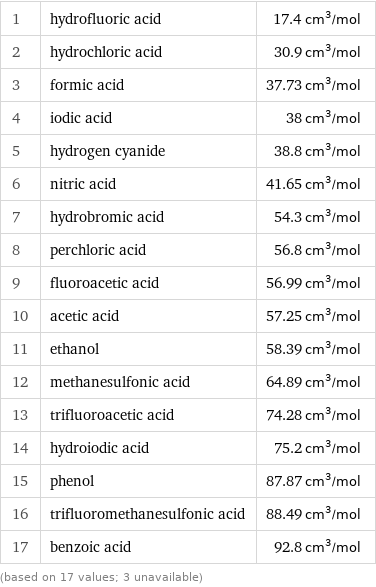
1 | hydrofluoric acid | 17.4 cm^3/mol 2 | hydrochloric acid | 30.9 cm^3/mol 3 | formic acid | 37.73 cm^3/mol 4 | iodic acid | 38 cm^3/mol 5 | hydrogen cyanide | 38.8 cm^3/mol 6 | nitric acid | 41.65 cm^3/mol 7 | hydrobromic acid | 54.3 cm^3/mol 8 | perchloric acid | 56.8 cm^3/mol 9 | fluoroacetic acid | 56.99 cm^3/mol 10 | acetic acid | 57.25 cm^3/mol 11 | ethanol | 58.39 cm^3/mol 12 | methanesulfonic acid | 64.89 cm^3/mol 13 | trifluoroacetic acid | 74.28 cm^3/mol 14 | hydroiodic acid | 75.2 cm^3/mol 15 | phenol | 87.87 cm^3/mol 16 | trifluoromethanesulfonic acid | 88.49 cm^3/mol 17 | benzoic acid | 92.8 cm^3/mol (based on 17 values; 3 unavailable)
Unit conversions for median molar volume 56.99 cm^3/mol
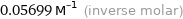
0.05699 M^(-1) (inverse molar)
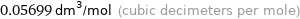
0.05699 dm^3/mol (cubic decimeters per mole)
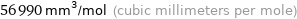
56990 mm^3/mol (cubic millimeters per mole)
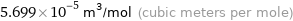
5.699×10^-5 m^3/mol (cubic meters per mole)
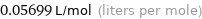
0.05699 L/mol (liters per mole)
Comparison for median molar volume 56.99 cm^3/mol

≈ ( 0.0025 ≈ 1/393 ) × molar volume of ideal gas at 273.15 K and 101.325 kPa ( 0.02241 m^3/mol )