Input interpretation
rhodium dioxide | potassium oxalate monohydrate
Chemical names and formulas
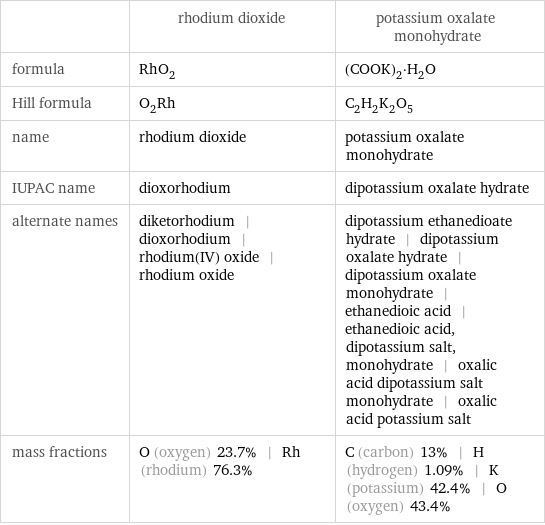
| rhodium dioxide | potassium oxalate monohydrate formula | RhO_2 | (COOK)_2·H_2O Hill formula | O_2Rh | C_2H_2K_2O_5 name | rhodium dioxide | potassium oxalate monohydrate IUPAC name | dioxorhodium | dipotassium oxalate hydrate alternate names | diketorhodium | dioxorhodium | rhodium(IV) oxide | rhodium oxide | dipotassium ethanedioate hydrate | dipotassium oxalate hydrate | dipotassium oxalate monohydrate | ethanedioic acid | ethanedioic acid, dipotassium salt, monohydrate | oxalic acid dipotassium salt monohydrate | oxalic acid potassium salt mass fractions | O (oxygen) 23.7% | Rh (rhodium) 76.3% | C (carbon) 13% | H (hydrogen) 1.09% | K (potassium) 42.4% | O (oxygen) 43.4%
Structure diagrams
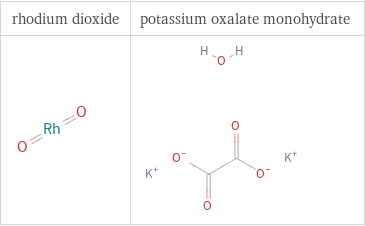
Structure diagrams
Basic properties
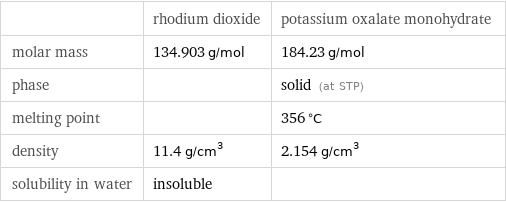
| rhodium dioxide | potassium oxalate monohydrate molar mass | 134.903 g/mol | 184.23 g/mol phase | | solid (at STP) melting point | | 356 °C density | 11.4 g/cm^3 | 2.154 g/cm^3 solubility in water | insoluble |
Units
