Input interpretation
potassium peroxide
Chemical names and formulas
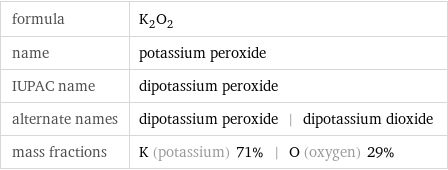
formula | K_2O_2 name | potassium peroxide IUPAC name | dipotassium peroxide alternate names | dipotassium peroxide | dipotassium dioxide mass fractions | K (potassium) 71% | O (oxygen) 29%
Structure diagram
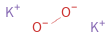
Structure diagram
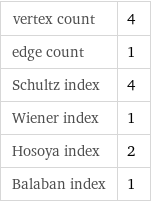
vertex count | 4 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
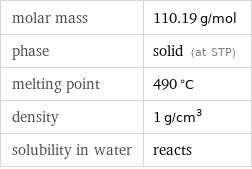
molar mass | 110.19 g/mol phase | solid (at STP) melting point | 490 °C density | 1 g/cm^3 solubility in water | reacts
Units

Solid properties (at STP)

density | 1 g/cm^3
Units

Thermodynamic properties
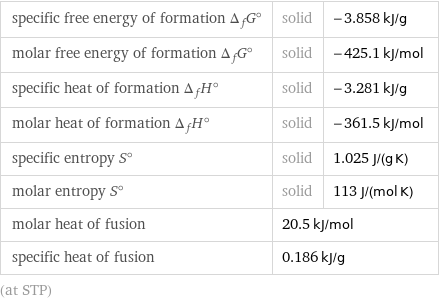
specific free energy of formation Δ_fG° | solid | -3.858 kJ/g molar free energy of formation Δ_fG° | solid | -425.1 kJ/mol specific heat of formation Δ_fH° | solid | -3.281 kJ/g molar heat of formation Δ_fH° | solid | -361.5 kJ/mol specific entropy S° | solid | 1.025 J/(g K) molar entropy S° | solid | 113 J/(mol K) molar heat of fusion | 20.5 kJ/mol | specific heat of fusion | 0.186 kJ/g | (at STP)
Chemical identifiers
![CAS number | 17014-71-0 PubChem CID number | 28202 SMILES identifier | [O-][O-].[K+].[K+] InChI identifier | InChI=1/2K.O2/c;;1-2/q2*+1;-2 EU number | 241-089-8 Gmelin number | 119841](../image_source/9a2e86db1ae5c1625a94a5fd4f7010c3.png)
CAS number | 17014-71-0 PubChem CID number | 28202 SMILES identifier | [O-][O-].[K+].[K+] InChI identifier | InChI=1/2K.O2/c;;1-2/q2*+1;-2 EU number | 241-089-8 Gmelin number | 119841
NFPA label

NFPA label
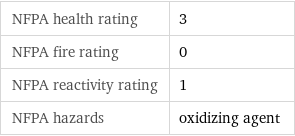
NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1 NFPA hazards | oxidizing agent
Ion equivalents

(O_2)^(2-) (peroxide anion) | 1 K^+ (potassium cation) | 2