Input interpretation
silver(I) sulfide | iron(II, III) oxide
Chemical names and formulas
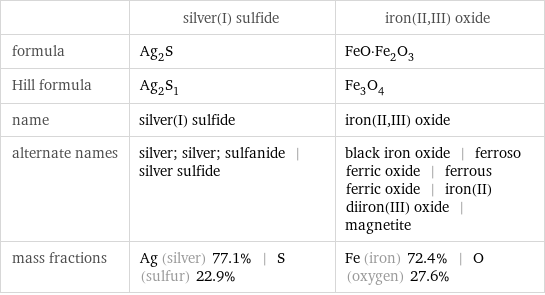
| silver(I) sulfide | iron(II, III) oxide formula | Ag_2S | FeO·Fe_2O_3 Hill formula | Ag_2S_1 | Fe_3O_4 name | silver(I) sulfide | iron(II, III) oxide alternate names | silver; silver; sulfanide | silver sulfide | black iron oxide | ferroso ferric oxide | ferrous ferric oxide | iron(II) diiron(III) oxide | magnetite mass fractions | Ag (silver) 77.1% | S (sulfur) 22.9% | Fe (iron) 72.4% | O (oxygen) 27.6%
Structure diagrams
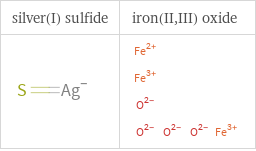
Structure diagrams
Basic properties
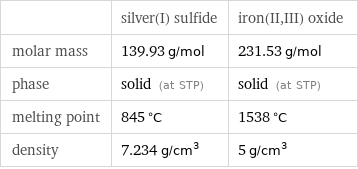
| silver(I) sulfide | iron(II, III) oxide molar mass | 139.93 g/mol | 231.53 g/mol phase | solid (at STP) | solid (at STP) melting point | 845 °C | 1538 °C density | 7.234 g/cm^3 | 5 g/cm^3
Units

Thermodynamic properties

| silver(I) sulfide | iron(II, III) oxide specific heat of fusion | 0.056 kJ/g (kilojoules per gram) | 0.596 kJ/g (kilojoules per gram)