Input interpretation
barium (chemical element) | lanthanum (chemical element) | holmium (chemical element) | terbium (chemical element)
Periodic table location
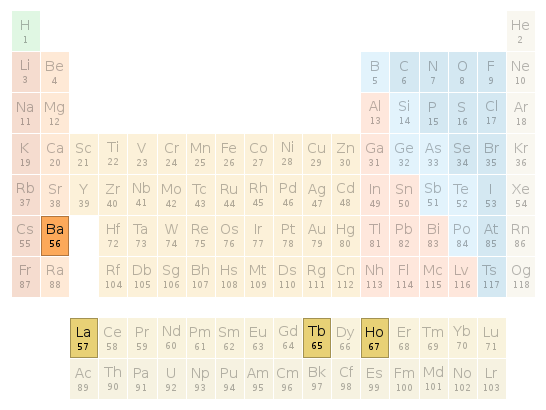
Periodic table location
Images
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration barium | Ba | 56 | [Xe]6s^2 lanthanum | La | 57 | [Xe]6s^25d^1 holmium | Ho | 67 | [Xe]6s^24f^11 terbium | Tb | 65 | [Xe]6s^24f^9 | Aufbau diagram | block | group barium | 6s | s | 2 lanthanum | 5d 6s | f | holmium | 4f 6s | f | terbium | 4f 6s | f | | period | atomic mass | half-life barium | 6 | 137.327 u | (stable) lanthanum | 6 | 138.90547 u | (stable) holmium | 6 | 164.93033 u | (stable) terbium | 6 | 158.92535 u | (stable)](../image_source/0d94b1284ce85e2ef9e8793553578606.png)
| atomic symbol | atomic number | short electronic configuration barium | Ba | 56 | [Xe]6s^2 lanthanum | La | 57 | [Xe]6s^25d^1 holmium | Ho | 67 | [Xe]6s^24f^11 terbium | Tb | 65 | [Xe]6s^24f^9 | Aufbau diagram | block | group barium | 6s | s | 2 lanthanum | 5d 6s | f | holmium | 4f 6s | f | terbium | 4f 6s | f | | period | atomic mass | half-life barium | 6 | 137.327 u | (stable) lanthanum | 6 | 138.90547 u | (stable) holmium | 6 | 164.93033 u | (stable) terbium | 6 | 158.92535 u | (stable)
Thermodynamic properties
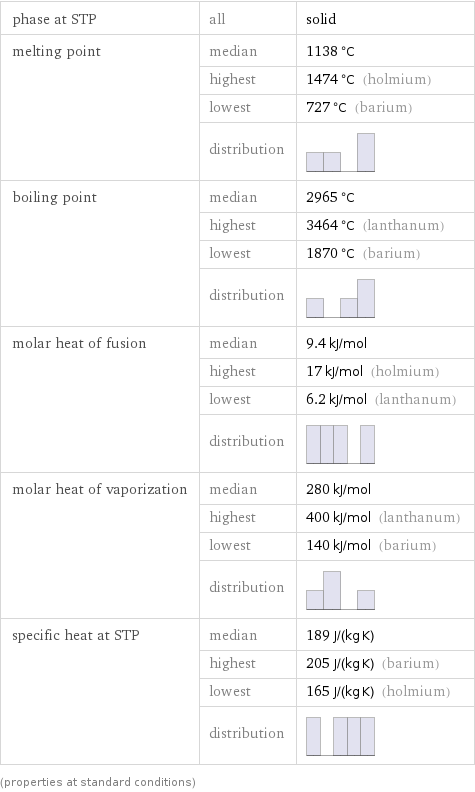
phase at STP | all | solid melting point | median | 1138 °C | highest | 1474 °C (holmium) | lowest | 727 °C (barium) | distribution | boiling point | median | 2965 °C | highest | 3464 °C (lanthanum) | lowest | 1870 °C (barium) | distribution | molar heat of fusion | median | 9.4 kJ/mol | highest | 17 kJ/mol (holmium) | lowest | 6.2 kJ/mol (lanthanum) | distribution | molar heat of vaporization | median | 280 kJ/mol | highest | 400 kJ/mol (lanthanum) | lowest | 140 kJ/mol (barium) | distribution | specific heat at STP | median | 189 J/(kg K) | highest | 205 J/(kg K) (barium) | lowest | 165 J/(kg K) (holmium) | distribution | (properties at standard conditions)
Material properties
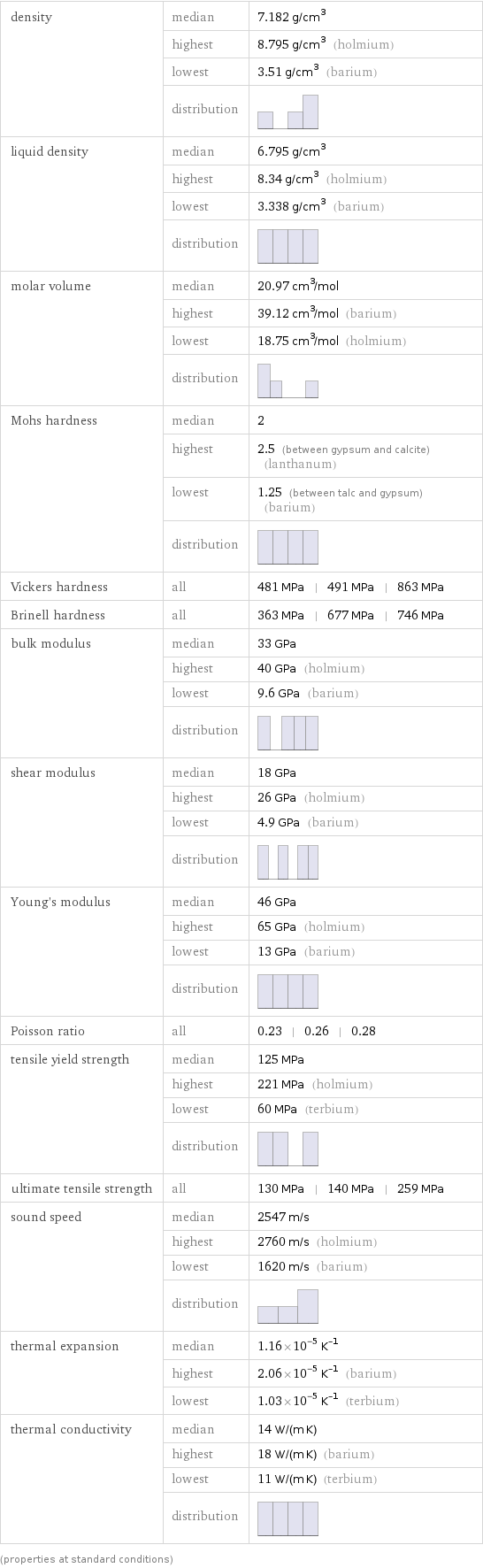
density | median | 7.182 g/cm^3 | highest | 8.795 g/cm^3 (holmium) | lowest | 3.51 g/cm^3 (barium) | distribution | liquid density | median | 6.795 g/cm^3 | highest | 8.34 g/cm^3 (holmium) | lowest | 3.338 g/cm^3 (barium) | distribution | molar volume | median | 20.97 cm^3/mol | highest | 39.12 cm^3/mol (barium) | lowest | 18.75 cm^3/mol (holmium) | distribution | Mohs hardness | median | 2 | highest | 2.5 (between gypsum and calcite) (lanthanum) | lowest | 1.25 (between talc and gypsum) (barium) | distribution | Vickers hardness | all | 481 MPa | 491 MPa | 863 MPa Brinell hardness | all | 363 MPa | 677 MPa | 746 MPa bulk modulus | median | 33 GPa | highest | 40 GPa (holmium) | lowest | 9.6 GPa (barium) | distribution | shear modulus | median | 18 GPa | highest | 26 GPa (holmium) | lowest | 4.9 GPa (barium) | distribution | Young's modulus | median | 46 GPa | highest | 65 GPa (holmium) | lowest | 13 GPa (barium) | distribution | Poisson ratio | all | 0.23 | 0.26 | 0.28 tensile yield strength | median | 125 MPa | highest | 221 MPa (holmium) | lowest | 60 MPa (terbium) | distribution | ultimate tensile strength | all | 130 MPa | 140 MPa | 259 MPa sound speed | median | 2547 m/s | highest | 2760 m/s (holmium) | lowest | 1620 m/s (barium) | distribution | thermal expansion | median | 1.16×10^-5 K^(-1) | highest | 2.06×10^-5 K^(-1) (barium) | lowest | 1.03×10^-5 K^(-1) (terbium) thermal conductivity | median | 14 W/(m K) | highest | 18 W/(m K) (barium) | lowest | 11 W/(m K) (terbium) | distribution | (properties at standard conditions)
Electromagnetic properties
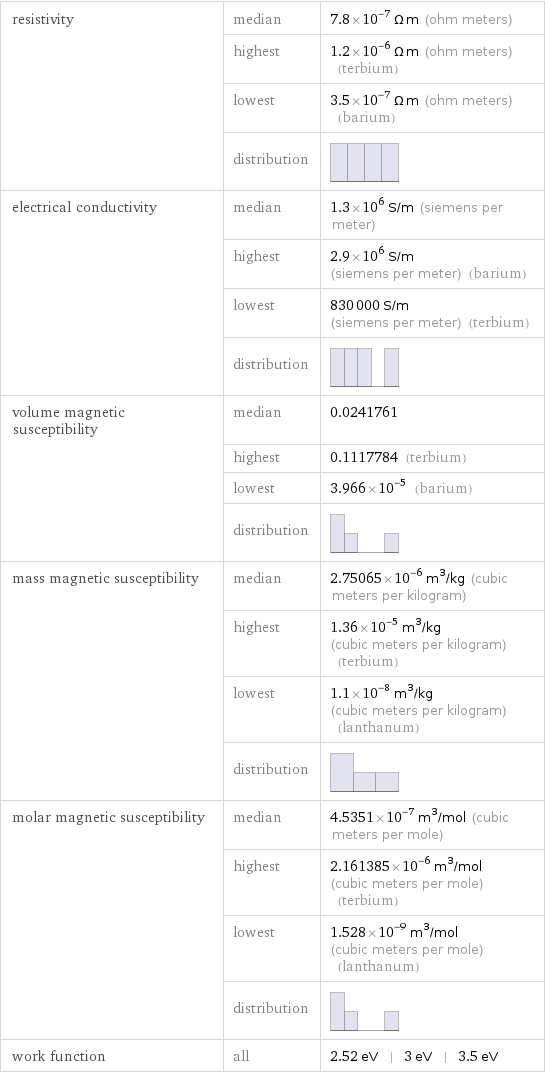
resistivity | median | 7.8×10^-7 Ω m (ohm meters) | highest | 1.2×10^-6 Ω m (ohm meters) (terbium) | lowest | 3.5×10^-7 Ω m (ohm meters) (barium) | distribution | electrical conductivity | median | 1.3×10^6 S/m (siemens per meter) | highest | 2.9×10^6 S/m (siemens per meter) (barium) | lowest | 830000 S/m (siemens per meter) (terbium) | distribution | volume magnetic susceptibility | median | 0.0241761 | highest | 0.1117784 (terbium) | lowest | 3.966×10^-5 (barium) | distribution | mass magnetic susceptibility | median | 2.75065×10^-6 m^3/kg (cubic meters per kilogram) | highest | 1.36×10^-5 m^3/kg (cubic meters per kilogram) (terbium) | lowest | 1.1×10^-8 m^3/kg (cubic meters per kilogram) (lanthanum) | distribution | molar magnetic susceptibility | median | 4.5351×10^-7 m^3/mol (cubic meters per mole) | highest | 2.161385×10^-6 m^3/mol (cubic meters per mole) (terbium) | lowest | 1.528×10^-9 m^3/mol (cubic meters per mole) (lanthanum) | distribution | work function | all | 2.52 eV | 3 eV | 3.5 eV
Reactivity
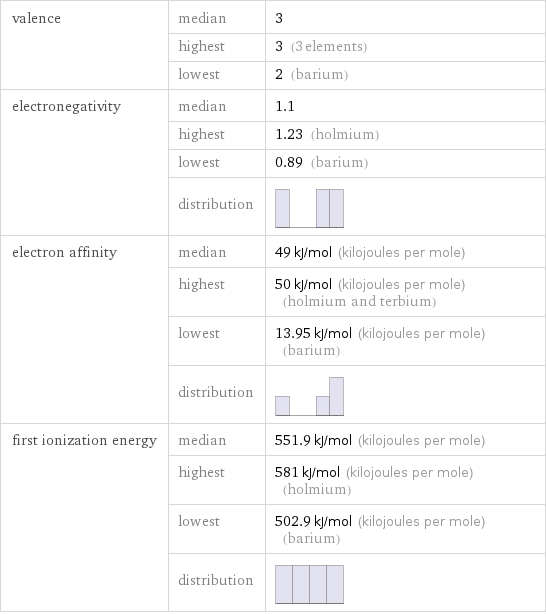
valence | median | 3 | highest | 3 (3 elements) | lowest | 2 (barium) electronegativity | median | 1.1 | highest | 1.23 (holmium) | lowest | 0.89 (barium) | distribution | electron affinity | median | 49 kJ/mol (kilojoules per mole) | highest | 50 kJ/mol (kilojoules per mole) (holmium and terbium) | lowest | 13.95 kJ/mol (kilojoules per mole) (barium) | distribution | first ionization energy | median | 551.9 kJ/mol (kilojoules per mole) | highest | 581 kJ/mol (kilojoules per mole) (holmium) | lowest | 502.9 kJ/mol (kilojoules per mole) (barium) | distribution |