Input interpretation
sodium hydrogen fluoride
Chemical names and formulas
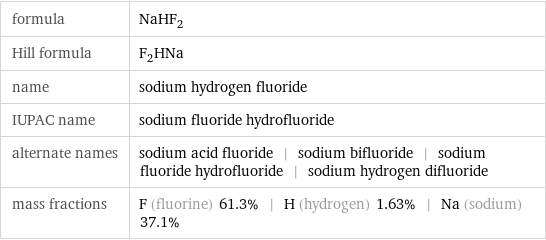
formula | NaHF_2 Hill formula | F_2HNa name | sodium hydrogen fluoride IUPAC name | sodium fluoride hydrofluoride alternate names | sodium acid fluoride | sodium bifluoride | sodium fluoride hydrofluoride | sodium hydrogen difluoride mass fractions | F (fluorine) 61.3% | H (hydrogen) 1.63% | Na (sodium) 37.1%
Structure diagram

Structure diagram
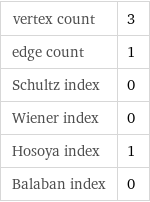
vertex count | 3 edge count | 1 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
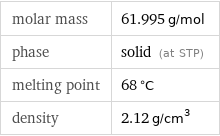
molar mass | 61.995 g/mol phase | solid (at STP) melting point | 68 °C density | 2.12 g/cm^3
Units

Solid properties (at STP)

density | 2.12 g/cm^3
Units

Thermodynamic properties
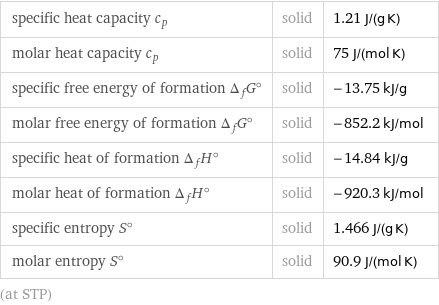
specific heat capacity c_p | solid | 1.21 J/(g K) molar heat capacity c_p | solid | 75 J/(mol K) specific free energy of formation Δ_fG° | solid | -13.75 kJ/g molar free energy of formation Δ_fG° | solid | -852.2 kJ/mol specific heat of formation Δ_fH° | solid | -14.84 kJ/g molar heat of formation Δ_fH° | solid | -920.3 kJ/mol specific entropy S° | solid | 1.466 J/(g K) molar entropy S° | solid | 90.9 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 1333-83-1 PubChem CID number | 219061 PubChem SID number | 24858779 SMILES identifier | F.[F-].[Na+] InChI identifier | InChI=1/2FH.Na/h2*1H;/q;;+1/p-1/fFH.F.Na/h;1h;/q;-1;m RTECS number | WB0350010 MDL number | MFCD00003529](../image_source/68fe4b434610e671db7f965d61922297.png)
CAS number | 1333-83-1 PubChem CID number | 219061 PubChem SID number | 24858779 SMILES identifier | F.[F-].[Na+] InChI identifier | InChI=1/2FH.Na/h2*1H;/q;;+1/p-1/fFH.F.Na/h;1h;/q;-1;m RTECS number | WB0350010 MDL number | MFCD00003529
Toxicity properties

RTECS classes | other
Ion equivalents

Na^+ (sodium cation) | 1 F^- (fluoride anion) | 1