Input interpretation

organic solvents | vapor pressure
Summary

median | 10651 Pa highest | 185972 Pa (N-pentane) lowest | 9.331 Pa (hexamethylphosphoric triamide) distribution |
Units

Distribution plots
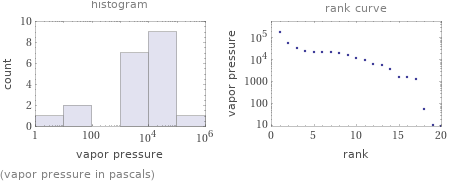
(vapor pressure in pascals)
Vapor pressure rankings
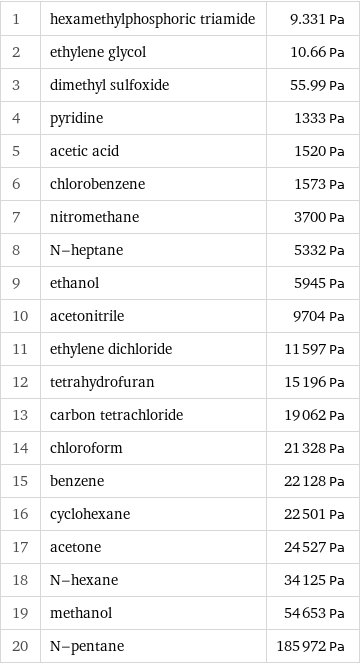
1 | hexamethylphosphoric triamide | 9.331 Pa 2 | ethylene glycol | 10.66 Pa 3 | dimethyl sulfoxide | 55.99 Pa 4 | pyridine | 1333 Pa 5 | acetic acid | 1520 Pa 6 | chlorobenzene | 1573 Pa 7 | nitromethane | 3700 Pa 8 | N-heptane | 5332 Pa 9 | ethanol | 5945 Pa 10 | acetonitrile | 9704 Pa 11 | ethylene dichloride | 11597 Pa 12 | tetrahydrofuran | 15196 Pa 13 | carbon tetrachloride | 19062 Pa 14 | chloroform | 21328 Pa 15 | benzene | 22128 Pa 16 | cyclohexane | 22501 Pa 17 | acetone | 24527 Pa 18 | N-hexane | 34125 Pa 19 | methanol | 54653 Pa 20 | N-pentane | 185972 Pa
Unit conversions for median vapor pressure 10651 Pa
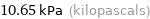
10.65 kPa (kilopascals)
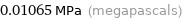
0.01065 MPa (megapascals)

10651 N/m^2 (newtons per square meter)
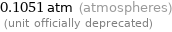
0.1051 atm (atmospheres) (unit officially deprecated)

0.1086 at (technical atmospheres) (unit officially deprecated)
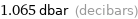
1.065 dbar (decibars)
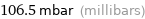
106.5 mbar (millibars)
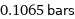
0.1065 bars
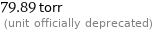
79.89 torr (unit officially deprecated)
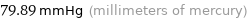
79.89 mmHg (millimeters of mercury)
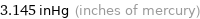
3.145 inHg (inches of mercury)
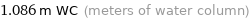
1.086 m WC (meters of water column)

108.6 cm WC (centimeters of water column)

42.76 in WC (inches of water column (conventional))
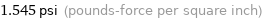
1.545 psi (pounds-force per square inch)
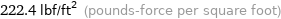
222.4 lbf/ft^2 (pounds-force per square foot)
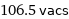
106.5 vacs
Comparisons for median vapor pressure 10651 Pa

≈ 0.67 × typical systolic overpressure in human arteries ( ≈ 120 mmHg )
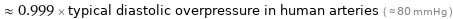
≈ 0.999 × typical diastolic overpressure in human arteries ( ≈ 80 mmHg )
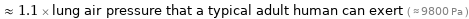
≈ 1.1 × lung air pressure that a typical adult human can exert ( ≈ 9800 Pa )
Corresponding quantities
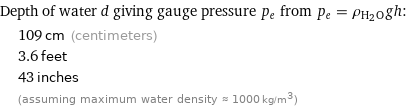
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 109 cm (centimeters) | 3.6 feet | 43 inches | (assuming maximum water density ≈ 1000 kg/m^3)

Standard Atmosphere altitude: | 16 km (kilometers) | 9.8 miles | (based on 1976 US Standard Atmosphere)