Input interpretation
ozone | iodine
Chemical names and formulas
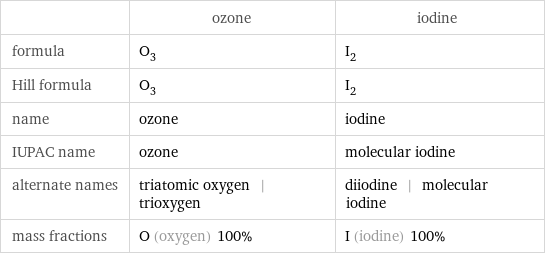
| ozone | iodine formula | O_3 | I_2 Hill formula | O_3 | I_2 name | ozone | iodine IUPAC name | ozone | molecular iodine alternate names | triatomic oxygen | trioxygen | diiodine | molecular iodine mass fractions | O (oxygen) 100% | I (iodine) 100%
Structure diagrams
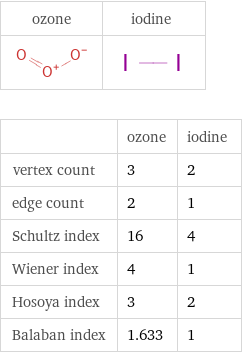
| ozone | iodine vertex count | 3 | 2 edge count | 2 | 1 Schultz index | 16 | 4 Wiener index | 4 | 1 Hosoya index | 3 | 2 Balaban index | 1.633 | 1
3D structure
3D structure
Basic properties
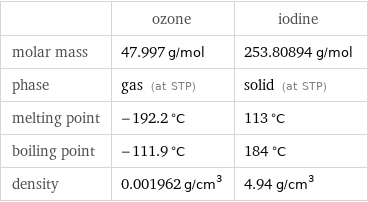
| ozone | iodine molar mass | 47.997 g/mol | 253.80894 g/mol phase | gas (at STP) | solid (at STP) melting point | -192.2 °C | 113 °C boiling point | -111.9 °C | 184 °C density | 0.001962 g/cm^3 | 4.94 g/cm^3
Units

Gas properties
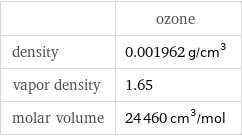
| ozone density | 0.001962 g/cm^3 vapor density | 1.65 molar volume | 24460 cm^3/mol
Units

Thermodynamic properties
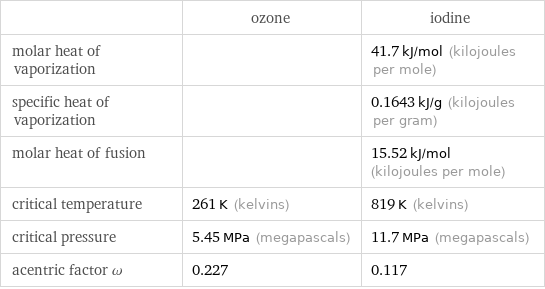
| ozone | iodine molar heat of vaporization | | 41.7 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.1643 kJ/g (kilojoules per gram) molar heat of fusion | | 15.52 kJ/mol (kilojoules per mole) critical temperature | 261 K (kelvins) | 819 K (kelvins) critical pressure | 5.45 MPa (megapascals) | 11.7 MPa (megapascals) acentric factor ω | 0.227 | 0.117
Solid properties
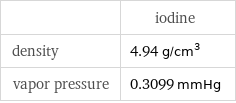
| iodine density | 4.94 g/cm^3 vapor pressure | 0.3099 mmHg
Units
