Input interpretation

freezing-point depression equation
Equation

ΔT_f = i K_f m | ΔT_f | freezing point depression K_f | cryoscopic constant m | solution molality i | van 't Hoff factor (assuming dilute ideal solutions)
Input values
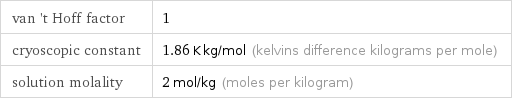
van 't Hoff factor | 1 cryoscopic constant | 1.86 K kg/mol (kelvins difference kilograms per mole) solution molality | 2 mol/kg (moles per kilogram)
Results
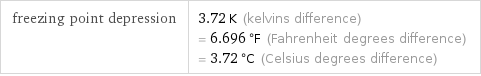
freezing point depression | 3.72 K (kelvins difference) = 6.696 °F (Fahrenheit degrees difference) = 3.72 °C (Celsius degrees difference)
Possible intermediate steps
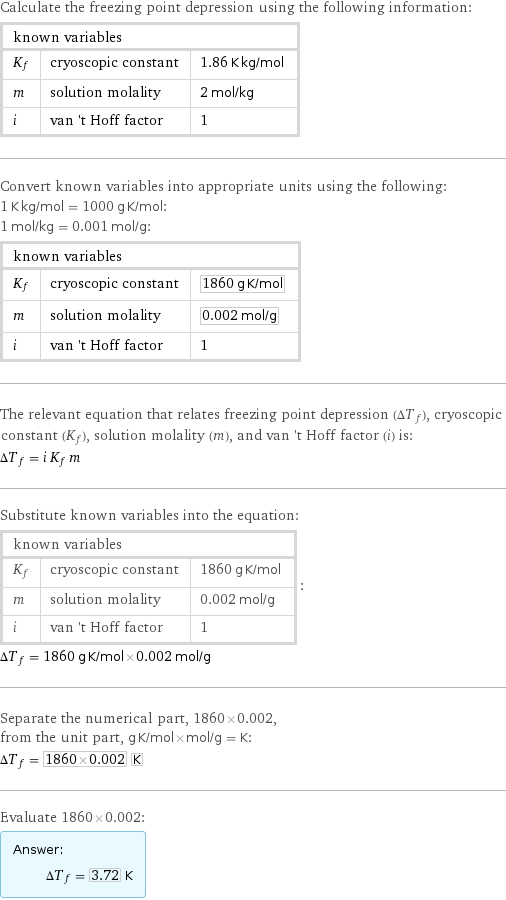
Calculate the freezing point depression using the following information: known variables | | K_f | cryoscopic constant | 1.86 K kg/mol m | solution molality | 2 mol/kg i | van 't Hoff factor | 1 Convert known variables into appropriate units using the following: 1 K kg/mol = 1000 g K/mol: 1 mol/kg = 0.001 mol/g: known variables | | K_f | cryoscopic constant | 1860 g K/mol m | solution molality | 0.002 mol/g i | van 't Hoff factor | 1 The relevant equation that relates freezing point depression (ΔT_f), cryoscopic constant (K_f), solution molality (m), and van 't Hoff factor (i) is: ΔT_f = i K_f m Substitute known variables into the equation: known variables | | K_f | cryoscopic constant | 1860 g K/mol m | solution molality | 0.002 mol/g i | van 't Hoff factor | 1 | : ΔT_f = 1860 g K/mol×0.002 mol/g Separate the numerical part, 1860×0.002, from the unit part, g K/mol×mol/g = K: ΔT_f = 1860×0.002 K Evaluate 1860×0.002: Answer: | | ΔT_f = 3.72 K