Input interpretation
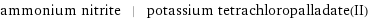
ammonium nitrite | potassium tetrachloropalladate(II)
Chemical names and formulas
![| ammonium nitrite | potassium tetrachloropalladate(II) formula | H_4N_2O_2 | K_2PdCl_4 Hill formula | H_4N_2O_2 | Cl_4K_2Pd name | ammonium nitrite | potassium tetrachloropalladate(II) IUPAC name | azanium nitrite | dipotassium tetrachloropalladium alternate names | ammonium nitrite [Forbidden] | nitrous acid, ammonium salt | dipotassium tetrachloropalladium | potassium palladium(II) chloride mass fractions | H (hydrogen) 6.3% | N (nitrogen) 43.7% | O (oxygen) 50% | Cl (chlorine) 43.4% | K (potassium) 24% | Pd (palladium) 32.6%](../image_source/f3c97de8d581f19b39144829c5438f06.png)
| ammonium nitrite | potassium tetrachloropalladate(II) formula | H_4N_2O_2 | K_2PdCl_4 Hill formula | H_4N_2O_2 | Cl_4K_2Pd name | ammonium nitrite | potassium tetrachloropalladate(II) IUPAC name | azanium nitrite | dipotassium tetrachloropalladium alternate names | ammonium nitrite [Forbidden] | nitrous acid, ammonium salt | dipotassium tetrachloropalladium | potassium palladium(II) chloride mass fractions | H (hydrogen) 6.3% | N (nitrogen) 43.7% | O (oxygen) 50% | Cl (chlorine) 43.4% | K (potassium) 24% | Pd (palladium) 32.6%
Structure diagrams
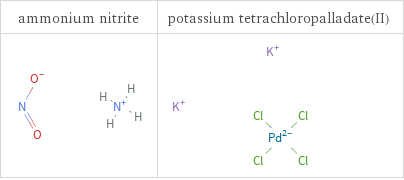
Structure diagrams
Basic properties
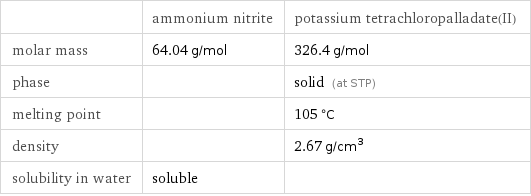
| ammonium nitrite | potassium tetrachloropalladate(II) molar mass | 64.04 g/mol | 326.4 g/mol phase | | solid (at STP) melting point | | 105 °C density | | 2.67 g/cm^3 solubility in water | soluble |
Units
