Input interpretation
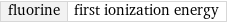
fluorine | first ionization energy
Result
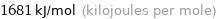
1681 kJ/mol (kilojoules per mole)
Periodic table location
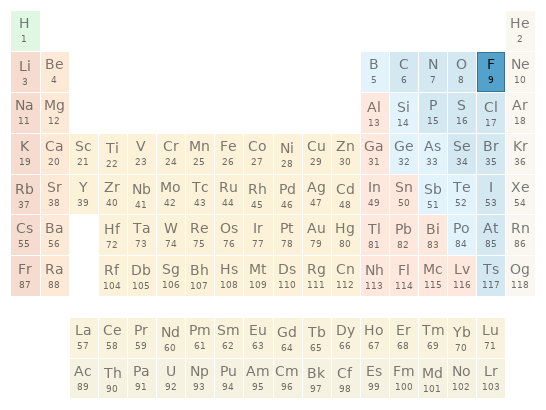
Periodic table location
Unit conversions
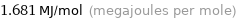
1.681 MJ/mol (megajoules per mole)
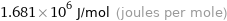
1.681×10^6 J/mol (joules per mole)

401.77 kcal/mol (thermochemical kilocalories per mole)

17.422 eV (molar electronvolts)
Comparisons as molar ionization energy

≈ 0.71 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
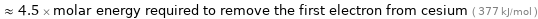
≈ 4.5 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )