Input interpretation

group 16 elements | atomic volume
Summary

median | 6.4×10^6 pm^3 highest | 2.87×10^7 pm^3 (polonium) lowest | 900000 pm^3 (oxygen) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
livermorium
Ranked values
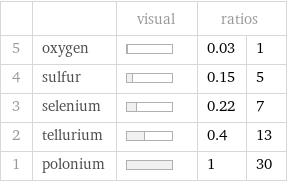
| | visual | ratios | 5 | oxygen | | 0.03 | 1 4 | sulfur | | 0.15 | 5 3 | selenium | | 0.22 | 7 2 | tellurium | | 0.4 | 13 1 | polonium | | 1 | 30
Plot
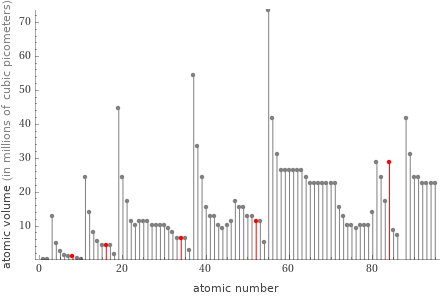
Plot
Periodic table location
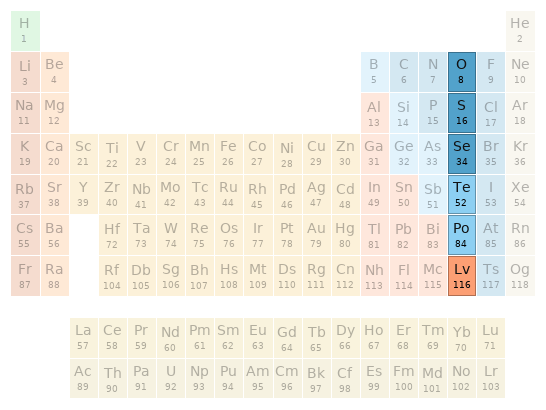
Periodic table location
Atomic radii properties
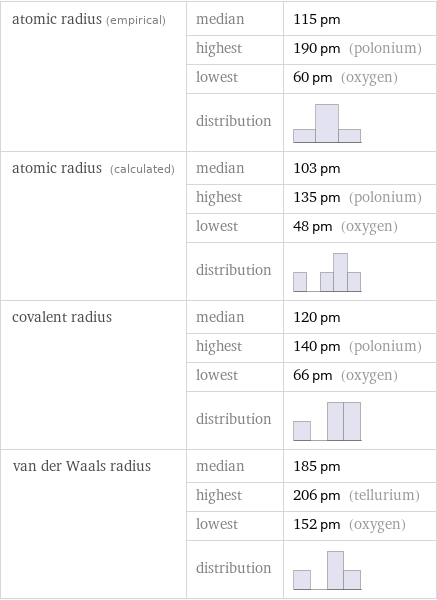
atomic radius (empirical) | median | 115 pm | highest | 190 pm (polonium) | lowest | 60 pm (oxygen) | distribution | atomic radius (calculated) | median | 103 pm | highest | 135 pm (polonium) | lowest | 48 pm (oxygen) | distribution | covalent radius | median | 120 pm | highest | 140 pm (polonium) | lowest | 66 pm (oxygen) | distribution | van der Waals radius | median | 185 pm | highest | 206 pm (tellurium) | lowest | 152 pm (oxygen) | distribution |
Units

Atomic volume rankings
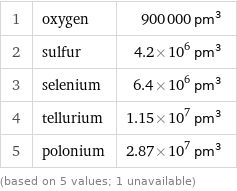
1 | oxygen | 900000 pm^3 2 | sulfur | 4.2×10^6 pm^3 3 | selenium | 6.4×10^6 pm^3 4 | tellurium | 1.15×10^7 pm^3 5 | polonium | 2.87×10^7 pm^3 (based on 5 values; 1 unavailable)
Unit conversions for median atomic volume 6.4×10^6 pm^3
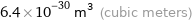
6.4×10^-30 m^3 (cubic meters)
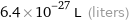
6.4×10^-27 L (liters)
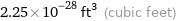
2.25×10^-28 ft^3 (cubic feet)
Comparisons for median atomic volume 6.4×10^6 pm^3

≈ 51 × volume of a helium atom ( 4 Pi/3 atomic radii of helium (the smallest atom)^3 )

≈ 97 × volume of a hydrogen atom ( 4 Pi/3 atomic radii of hydrogen^3 )
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 115 pm (picometers) | 4.5×10^-9 inches

Edge length a of a cube from V = a^3: | 185 pm (picometers) | 7.3×10^-9 inches