Input interpretation

elements | first molar ionization energy
Summary
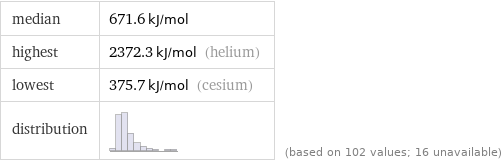
median | 671.6 kJ/mol highest | 2372.3 kJ/mol (helium) lowest | 375.7 kJ/mol (cesium) distribution | | (based on 102 values; 16 unavailable)
Entities with missing values
lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium | ... (total: 16)
Distribution plots
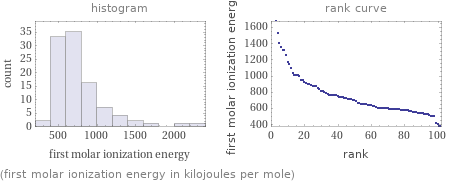
(first molar ionization energy in kilojoules per mole)
First molar ionization energy rankings

1 | cesium | 375.7 kJ/mol 2 | francium | 380 kJ/mol 3 | rubidium | 403 kJ/mol 4 | potassium | 418.8 kJ/mol 5 | sodium | 495.8 kJ/mol 6 | actinium | 499 kJ/mol 7 | barium | 502.9 kJ/mol 8 | radium | 509.3 kJ/mol 9 | lithium | 520.2 kJ/mol 10 | lutetium | 523.5 kJ/mol ⋮ | | 93 | xenon | 1170.4 kJ/mol 94 | chlorine | 1251.2 kJ/mol 95 | hydrogen | 1312 kJ/mol 96 | oxygen | 1313.9 kJ/mol 97 | krypton | 1350.8 kJ/mol 98 | nitrogen | 1402.3 kJ/mol 99 | argon | 1520.6 kJ/mol 100 | fluorine | 1681 kJ/mol 101 | neon | 2080.7 kJ/mol 102 | helium | 2372.3 kJ/mol (based on 102 values; 16 unavailable)
Unit conversions for median first molar ionization energy 671.6 kJ/mol
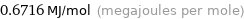
0.6716 MJ/mol (megajoules per mole)
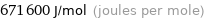
671600 J/mol (joules per mole)
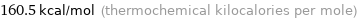
160.5 kcal/mol (thermochemical kilocalories per mole)
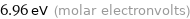
6.96 eV (molar electronvolts)
Comparisons for median first molar ionization energy 671.6 kJ/mol

≈ 0.28 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
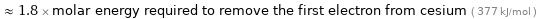
≈ 1.8 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )