Input interpretation

sodium sulfate heptahydrate | mercury(I) bromide
Chemical names and formulas
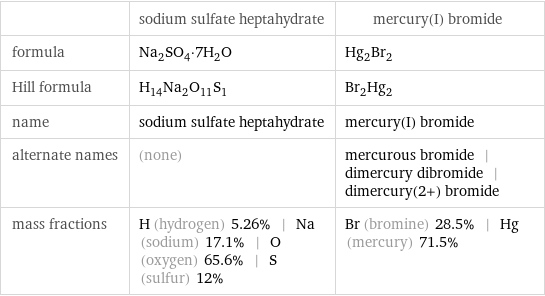
| sodium sulfate heptahydrate | mercury(I) bromide formula | Na_2SO_4·7H_2O | Hg_2Br_2 Hill formula | H_14Na_2O_11S_1 | Br_2Hg_2 name | sodium sulfate heptahydrate | mercury(I) bromide alternate names | (none) | mercurous bromide | dimercury dibromide | dimercury(2+) bromide mass fractions | H (hydrogen) 5.26% | Na (sodium) 17.1% | O (oxygen) 65.6% | S (sulfur) 12% | Br (bromine) 28.5% | Hg (mercury) 71.5%
Structure diagrams
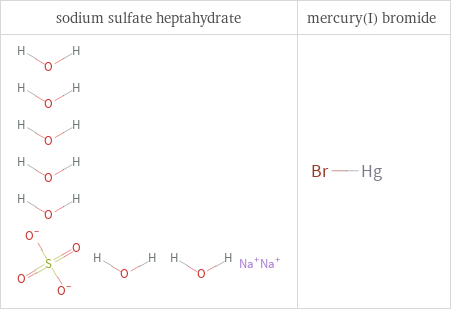
Structure diagrams
Basic properties

| sodium sulfate heptahydrate | mercury(I) bromide molar mass | 268.14 g/mol | 280.5 g/mol phase | | solid (at STP) melting point | | 445 °C density | | 7.31 g/cm^3 solubility in water | soluble | insoluble
Units
