Input interpretation

non-polar solvents | specific heat of vaporization
Summary
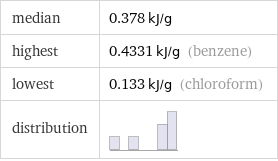
median | 0.378 kJ/g highest | 0.4331 kJ/g (benzene) lowest | 0.133 kJ/g (chloroform) distribution |
Units

Ranked values
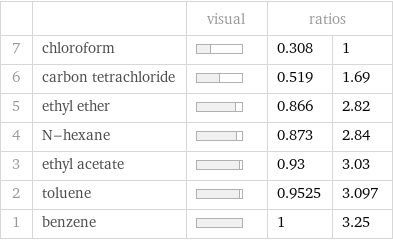
| | visual | ratios | 7 | chloroform | | 0.308 | 1 6 | carbon tetrachloride | | 0.519 | 1.69 5 | ethyl ether | | 0.866 | 2.82 4 | N-hexane | | 0.873 | 2.84 3 | ethyl acetate | | 0.93 | 3.03 2 | toluene | | 0.9525 | 3.097 1 | benzene | | 1 | 3.25
Specific heat of vaporization rankings
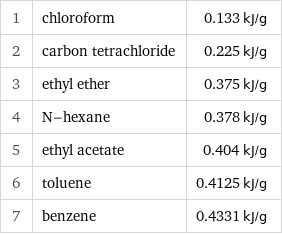
1 | chloroform | 0.133 kJ/g 2 | carbon tetrachloride | 0.225 kJ/g 3 | ethyl ether | 0.375 kJ/g 4 | N-hexane | 0.378 kJ/g 5 | ethyl acetate | 0.404 kJ/g 6 | toluene | 0.4125 kJ/g 7 | benzene | 0.4331 kJ/g
Unit conversions for median specific heat of vaporization 0.378 kJ/g

378 J/g (joules per gram)
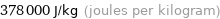
378000 J/kg (joules per kilogram)
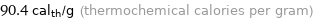
90.4 cal_th/g (thermochemical calories per gram)
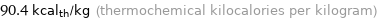
90.4 kcal_th/kg (thermochemical kilocalories per kilogram)
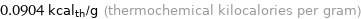
0.0904 kcal_th/g (thermochemical kilocalories per gram)

163 BTU_IT/lb (IT British thermal units per pound)