Input interpretation

carbon lead | melting point
Results
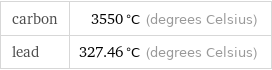
carbon | 3550 °C (degrees Celsius) lead | 327.46 °C (degrees Celsius)
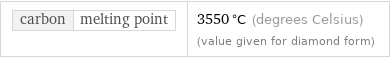
carbon | melting point | 3550 °C (degrees Celsius) (value given for diamond form)
Plot
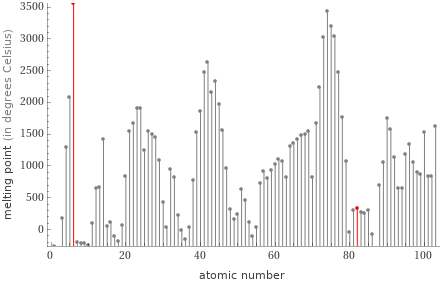
Plot
Thermodynamic properties
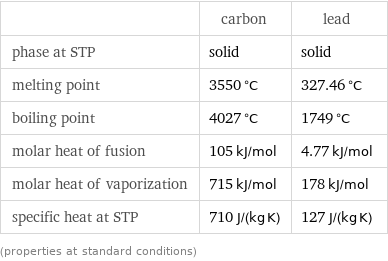
| carbon | lead phase at STP | solid | solid melting point | 3550 °C | 327.46 °C boiling point | 4027 °C | 1749 °C molar heat of fusion | 105 kJ/mol | 4.77 kJ/mol molar heat of vaporization | 715 kJ/mol | 178 kJ/mol specific heat at STP | 710 J/(kg K) | 127 J/(kg K) (properties at standard conditions)