Input interpretation
potassium iodide | ammonium nitrate
Chemical names and formulas
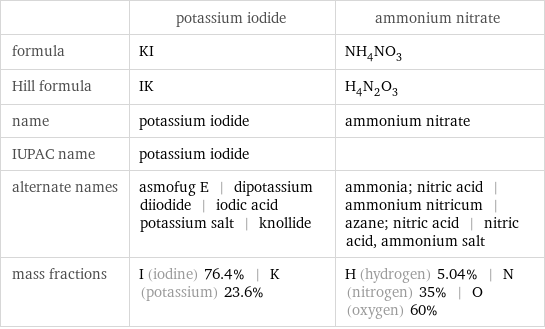
| potassium iodide | ammonium nitrate formula | KI | NH_4NO_3 Hill formula | IK | H_4N_2O_3 name | potassium iodide | ammonium nitrate IUPAC name | potassium iodide | alternate names | asmofug E | dipotassium diiodide | iodic acid potassium salt | knollide | ammonia; nitric acid | ammonium nitricum | azane; nitric acid | nitric acid, ammonium salt mass fractions | I (iodine) 76.4% | K (potassium) 23.6% | H (hydrogen) 5.04% | N (nitrogen) 35% | O (oxygen) 60%
Structure diagrams
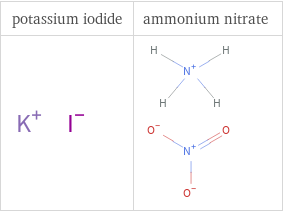
Structure diagrams
Basic properties
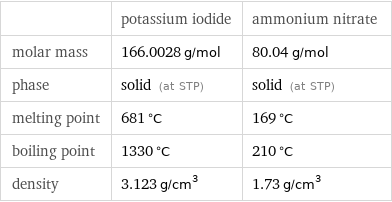
| potassium iodide | ammonium nitrate molar mass | 166.0028 g/mol | 80.04 g/mol phase | solid (at STP) | solid (at STP) melting point | 681 °C | 169 °C boiling point | 1330 °C | 210 °C density | 3.123 g/cm^3 | 1.73 g/cm^3
Units

Thermodynamic properties
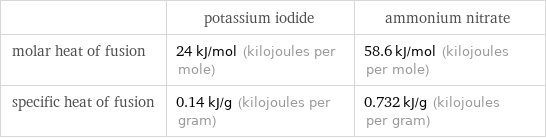
| potassium iodide | ammonium nitrate molar heat of fusion | 24 kJ/mol (kilojoules per mole) | 58.6 kJ/mol (kilojoules per mole) specific heat of fusion | 0.14 kJ/g (kilojoules per gram) | 0.732 kJ/g (kilojoules per gram)
Solid properties (at STP)
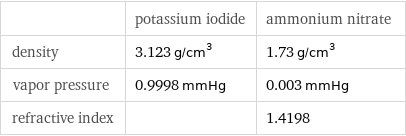
| potassium iodide | ammonium nitrate density | 3.123 g/cm^3 | 1.73 g/cm^3 vapor pressure | 0.9998 mmHg | 0.003 mmHg refractive index | | 1.4198
Units
