Input interpretation

polar protic solvents | specific heat of fusion
Summary
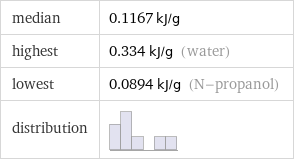
median | 0.1167 kJ/g highest | 0.334 kJ/g (water) lowest | 0.0894 kJ/g (N-propanol) distribution |
Units

Ranked values
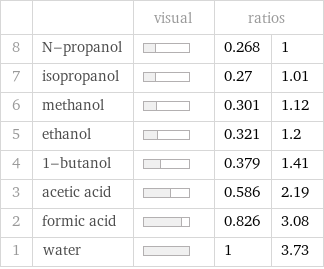
| | visual | ratios | 8 | N-propanol | | 0.268 | 1 7 | isopropanol | | 0.27 | 1.01 6 | methanol | | 0.301 | 1.12 5 | ethanol | | 0.321 | 1.2 4 | 1-butanol | | 0.379 | 1.41 3 | acetic acid | | 0.586 | 2.19 2 | formic acid | | 0.826 | 3.08 1 | water | | 1 | 3.73
Specific heat of fusion rankings
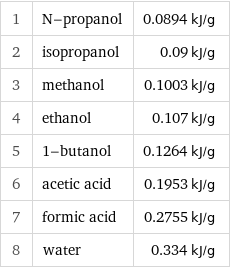
1 | N-propanol | 0.0894 kJ/g 2 | isopropanol | 0.09 kJ/g 3 | methanol | 0.1003 kJ/g 4 | ethanol | 0.107 kJ/g 5 | 1-butanol | 0.1264 kJ/g 6 | acetic acid | 0.1953 kJ/g 7 | formic acid | 0.2755 kJ/g 8 | water | 0.334 kJ/g
Unit conversions for median specific heat of fusion 0.1167 kJ/g

116.7 J/g (joules per gram)
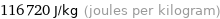
116720 J/kg (joules per kilogram)
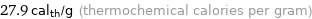
27.9 cal_th/g (thermochemical calories per gram)
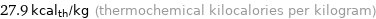
27.9 kcal_th/kg (thermochemical kilocalories per kilogram)
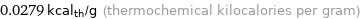
0.0279 kcal_th/g (thermochemical kilocalories per gram)

50.18 BTU_IT/lb (IT British thermal units per pound)