Input interpretation
sulfuric acid | sodium chloride
Chemical names and formulas
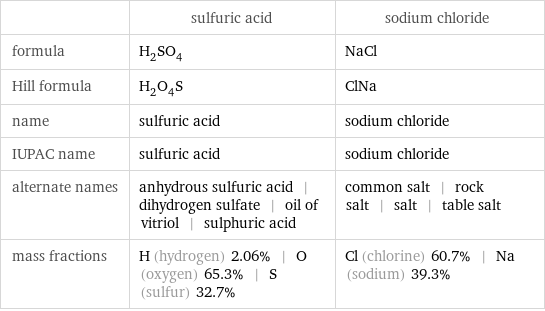
| sulfuric acid | sodium chloride formula | H_2SO_4 | NaCl Hill formula | H_2O_4S | ClNa name | sulfuric acid | sodium chloride IUPAC name | sulfuric acid | sodium chloride alternate names | anhydrous sulfuric acid | dihydrogen sulfate | oil of vitriol | sulphuric acid | common salt | rock salt | salt | table salt mass fractions | H (hydrogen) 2.06% | O (oxygen) 65.3% | S (sulfur) 32.7% | Cl (chlorine) 60.7% | Na (sodium) 39.3%
Structure diagrams
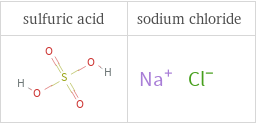
Structure diagrams
3D structure
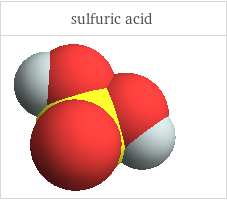
3D structure
Basic properties
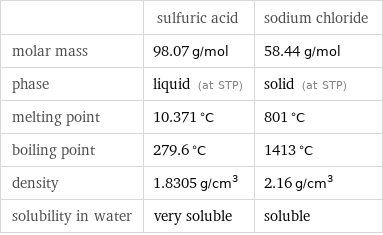
| sulfuric acid | sodium chloride molar mass | 98.07 g/mol | 58.44 g/mol phase | liquid (at STP) | solid (at STP) melting point | 10.371 °C | 801 °C boiling point | 279.6 °C | 1413 °C density | 1.8305 g/cm^3 | 2.16 g/cm^3 solubility in water | very soluble | soluble
Units

Liquid properties
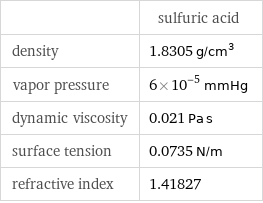
| sulfuric acid density | 1.8305 g/cm^3 vapor pressure | 6×10^-5 mmHg dynamic viscosity | 0.021 Pa s surface tension | 0.0735 N/m refractive index | 1.41827
Units
Thermodynamic properties
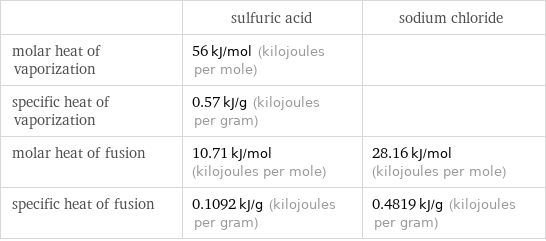
| sulfuric acid | sodium chloride molar heat of vaporization | 56 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.57 kJ/g (kilojoules per gram) | molar heat of fusion | 10.71 kJ/mol (kilojoules per mole) | 28.16 kJ/mol (kilojoules per mole) specific heat of fusion | 0.1092 kJ/g (kilojoules per gram) | 0.4819 kJ/g (kilojoules per gram)
Solid properties
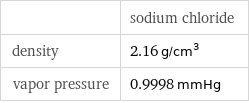
| sodium chloride density | 2.16 g/cm^3 vapor pressure | 0.9998 mmHg
Units
