Input interpretation
nitrogen trifluoride
Chemical names and formulas

formula | F_3N name | nitrogen trifluoride alternate names | nitrogen fluoride | nitrogen fluoride (1:3) | perfluoroammonia | trifluoroamine mass fractions | F (fluorine) 80.3% | N (nitrogen) 19.7%
Lewis structure
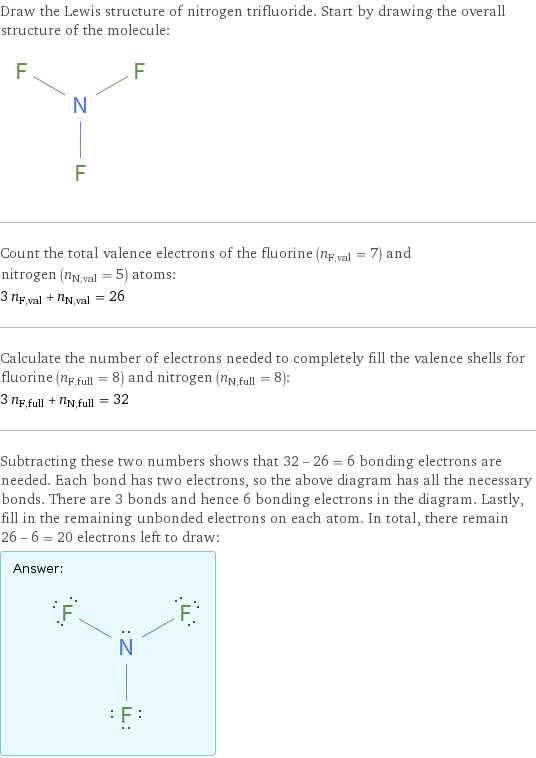
Draw the Lewis structure of nitrogen trifluoride. Start by drawing the overall structure of the molecule: Count the total valence electrons of the fluorine (n_F, val = 7) and nitrogen (n_N, val = 5) atoms: 3 n_F, val + n_N, val = 26 Calculate the number of electrons needed to completely fill the valence shells for fluorine (n_F, full = 8) and nitrogen (n_N, full = 8): 3 n_F, full + n_N, full = 32 Subtracting these two numbers shows that 32 - 26 = 6 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 3 bonds and hence 6 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 26 - 6 = 20 electrons left to draw: Answer: | |
3D structure

3D structure
Basic properties
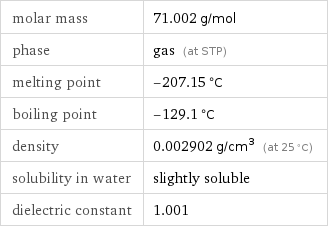
molar mass | 71.002 g/mol phase | gas (at STP) melting point | -207.15 °C boiling point | -129.1 °C density | 0.002902 g/cm^3 (at 25 °C) solubility in water | slightly soluble dielectric constant | 1.001
Gas properties (at STP)
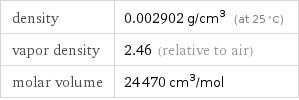
density | 0.002902 g/cm^3 (at 25 °C) vapor density | 2.46 (relative to air) molar volume | 24470 cm^3/mol
Units

Thermodynamic properties
specific heat capacity c_p | gas | 0.7521 J/(g K) molar heat capacity c_p | gas | 53.4 J/(mol K) specific free energy of formation Δ_fG° | gas | -1.276 kJ/g molar free energy of formation Δ_fG° | gas | -90.6 kJ/mol specific heat of formation Δ_fH° | gas | -1.861 kJ/g molar heat of formation Δ_fH° | gas | -132.1 kJ/mol specific entropy S° | gas | 3.673 J/(g K) molar entropy S° | gas | 260.8 J/(mol K) molar heat of vaporization | 11.59 kJ/mol | specific heat of vaporization | 0.1632 kJ/g | critical temperature | 230 K | critical pressure | 4.53 MPa | (at STP)
Phase diagram
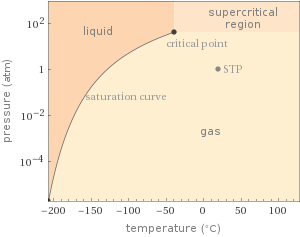
Phase diagram
Units

Chemical identifiers
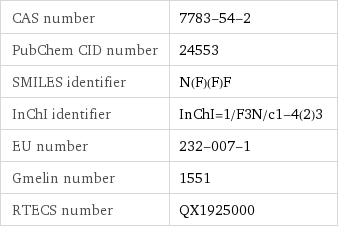
CAS number | 7783-54-2 PubChem CID number | 24553 SMILES identifier | N(F)(F)F InChI identifier | InChI=1/F3N/c1-4(2)3 EU number | 232-007-1 Gmelin number | 1551 RTECS number | QX1925000
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 3
Toxicity properties

short-term exposure limit | 45 mg/m^3

long-term exposure limit | 30 mg/m^3 (over 8 hours) RTECS classes | other