Input interpretation
beryllium (chemical element) | magnesium (chemical element)
Periodic table location
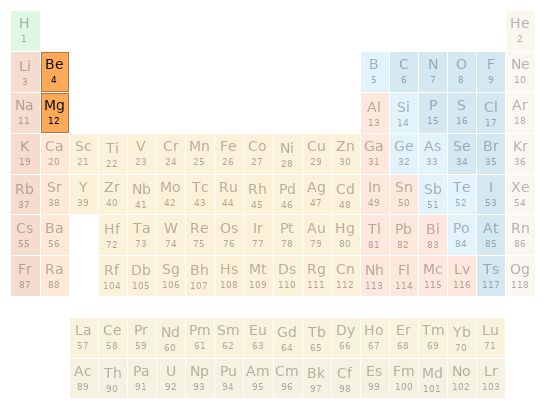
Periodic table location
Images
Images
Basic elemental properties
![| beryllium | magnesium atomic symbol | Be | Mg atomic number | 4 | 12 short electronic configuration | [He]2s^2 | [Ne]3s^2 Aufbau diagram | 2s | 3s block | s | s group | 2 | 2 period | 2 | 3 atomic mass | 9.0121831 u | 24.305 u half-life | (stable) | (stable)](../image_source/a6e9189a1122ec057f0176194041d63c.png)
| beryllium | magnesium atomic symbol | Be | Mg atomic number | 4 | 12 short electronic configuration | [He]2s^2 | [Ne]3s^2 Aufbau diagram | 2s | 3s block | s | s group | 2 | 2 period | 2 | 3 atomic mass | 9.0121831 u | 24.305 u half-life | (stable) | (stable)
Thermodynamic properties
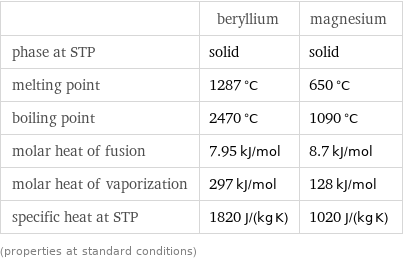
| beryllium | magnesium phase at STP | solid | solid melting point | 1287 °C | 650 °C boiling point | 2470 °C | 1090 °C molar heat of fusion | 7.95 kJ/mol | 8.7 kJ/mol molar heat of vaporization | 297 kJ/mol | 128 kJ/mol specific heat at STP | 1820 J/(kg K) | 1020 J/(kg K) (properties at standard conditions)
Material properties
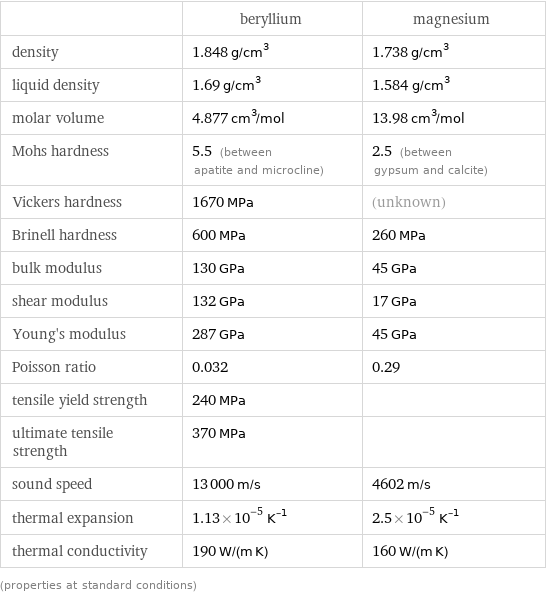
| beryllium | magnesium density | 1.848 g/cm^3 | 1.738 g/cm^3 liquid density | 1.69 g/cm^3 | 1.584 g/cm^3 molar volume | 4.877 cm^3/mol | 13.98 cm^3/mol Mohs hardness | 5.5 (between apatite and microcline) | 2.5 (between gypsum and calcite) Vickers hardness | 1670 MPa | (unknown) Brinell hardness | 600 MPa | 260 MPa bulk modulus | 130 GPa | 45 GPa shear modulus | 132 GPa | 17 GPa Young's modulus | 287 GPa | 45 GPa Poisson ratio | 0.032 | 0.29 tensile yield strength | 240 MPa | ultimate tensile strength | 370 MPa | sound speed | 13000 m/s | 4602 m/s thermal expansion | 1.13×10^-5 K^(-1) | 2.5×10^-5 K^(-1) thermal conductivity | 190 W/(m K) | 160 W/(m K) (properties at standard conditions)
Electromagnetic properties
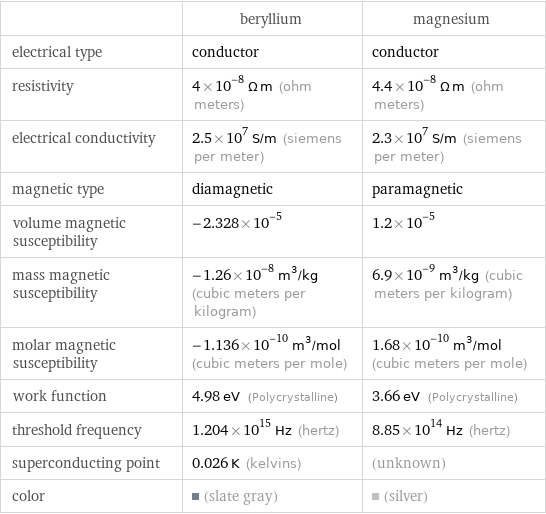
| beryllium | magnesium electrical type | conductor | conductor resistivity | 4×10^-8 Ω m (ohm meters) | 4.4×10^-8 Ω m (ohm meters) electrical conductivity | 2.5×10^7 S/m (siemens per meter) | 2.3×10^7 S/m (siemens per meter) magnetic type | diamagnetic | paramagnetic volume magnetic susceptibility | -2.328×10^-5 | 1.2×10^-5 mass magnetic susceptibility | -1.26×10^-8 m^3/kg (cubic meters per kilogram) | 6.9×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -1.136×10^-10 m^3/mol (cubic meters per mole) | 1.68×10^-10 m^3/mol (cubic meters per mole) work function | 4.98 eV (Polycrystalline) | 3.66 eV (Polycrystalline) threshold frequency | 1.204×10^15 Hz (hertz) | 8.85×10^14 Hz (hertz) superconducting point | 0.026 K (kelvins) | (unknown) color | (slate gray) | (silver)
Reactivity
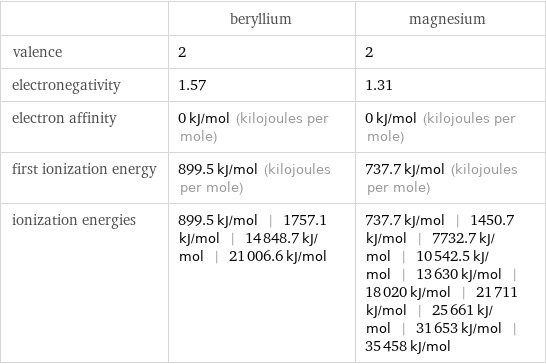
| beryllium | magnesium valence | 2 | 2 electronegativity | 1.57 | 1.31 electron affinity | 0 kJ/mol (kilojoules per mole) | 0 kJ/mol (kilojoules per mole) first ionization energy | 899.5 kJ/mol (kilojoules per mole) | 737.7 kJ/mol (kilojoules per mole) ionization energies | 899.5 kJ/mol | 1757.1 kJ/mol | 14848.7 kJ/mol | 21006.6 kJ/mol | 737.7 kJ/mol | 1450.7 kJ/mol | 7732.7 kJ/mol | 10542.5 kJ/mol | 13630 kJ/mol | 18020 kJ/mol | 21711 kJ/mol | 25661 kJ/mol | 31653 kJ/mol | 35458 kJ/mol
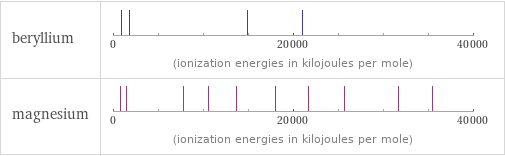
Reactivity
Atomic properties
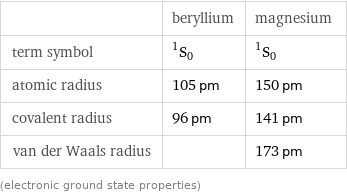
| beryllium | magnesium term symbol | ^1S_0 | ^1S_0 atomic radius | 105 pm | 150 pm covalent radius | 96 pm | 141 pm van der Waals radius | | 173 pm (electronic ground state properties)
Abundances
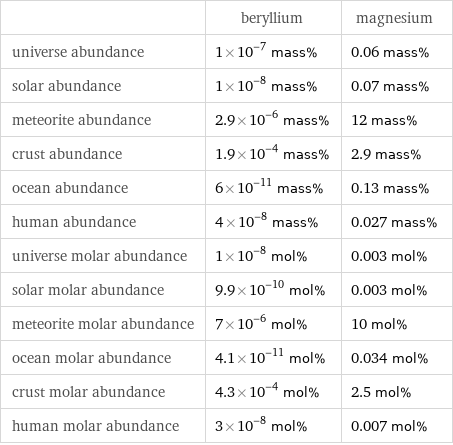
| beryllium | magnesium universe abundance | 1×10^-7 mass% | 0.06 mass% solar abundance | 1×10^-8 mass% | 0.07 mass% meteorite abundance | 2.9×10^-6 mass% | 12 mass% crust abundance | 1.9×10^-4 mass% | 2.9 mass% ocean abundance | 6×10^-11 mass% | 0.13 mass% human abundance | 4×10^-8 mass% | 0.027 mass% universe molar abundance | 1×10^-8 mol% | 0.003 mol% solar molar abundance | 9.9×10^-10 mol% | 0.003 mol% meteorite molar abundance | 7×10^-6 mol% | 10 mol% ocean molar abundance | 4.1×10^-11 mol% | 0.034 mol% crust molar abundance | 4.3×10^-4 mol% | 2.5 mol% human molar abundance | 3×10^-8 mol% | 0.007 mol%
Nuclear properties
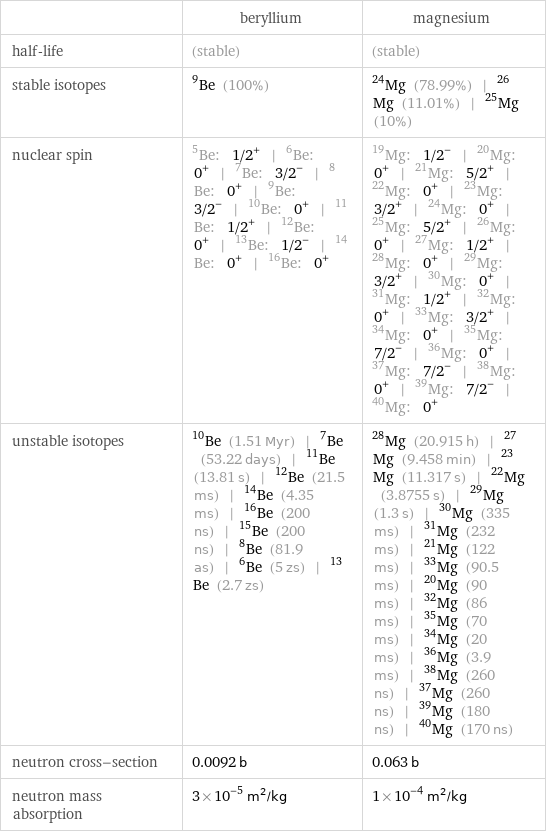
| beryllium | magnesium half-life | (stable) | (stable) stable isotopes | Be-9 (100%) | Mg-24 (78.99%) | Mg-26 (11.01%) | Mg-25 (10%) nuclear spin | Be-5: 1/2^+ | Be-6: 0^+ | Be-7: 3/2^- | Be-8: 0^+ | Be-9: 3/2^- | Be-10: 0^+ | Be-11: 1/2^+ | Be-12: 0^+ | Be-13: 1/2^- | Be-14: 0^+ | Be-16: 0^+ | Mg-19: 1/2^- | Mg-20: 0^+ | Mg-21: 5/2^+ | Mg-22: 0^+ | Mg-23: 3/2^+ | Mg-24: 0^+ | Mg-25: 5/2^+ | Mg-26: 0^+ | Mg-27: 1/2^+ | Mg-28: 0^+ | Mg-29: 3/2^+ | Mg-30: 0^+ | Mg-31: 1/2^+ | Mg-32: 0^+ | Mg-33: 3/2^+ | Mg-34: 0^+ | Mg-35: 7/2^- | Mg-36: 0^+ | Mg-37: 7/2^- | Mg-38: 0^+ | Mg-39: 7/2^- | Mg-40: 0^+ unstable isotopes | Be-10 (1.51 Myr) | Be-7 (53.22 days) | Be-11 (13.81 s) | Be-12 (21.5 ms) | Be-14 (4.35 ms) | Be-16 (200 ns) | Be-15 (200 ns) | Be-8 (81.9 as) | Be-6 (5 zs) | Be-13 (2.7 zs) | Mg-28 (20.915 h) | Mg-27 (9.458 min) | Mg-23 (11.317 s) | Mg-22 (3.8755 s) | Mg-29 (1.3 s) | Mg-30 (335 ms) | Mg-31 (232 ms) | Mg-21 (122 ms) | Mg-33 (90.5 ms) | Mg-20 (90 ms) | Mg-32 (86 ms) | Mg-35 (70 ms) | Mg-34 (20 ms) | Mg-36 (3.9 ms) | Mg-38 (260 ns) | Mg-37 (260 ns) | Mg-39 (180 ns) | Mg-40 (170 ns) neutron cross-section | 0.0092 b | 0.063 b neutron mass absorption | 3×10^-5 m^2/kg | 1×10^-4 m^2/kg
Identifiers
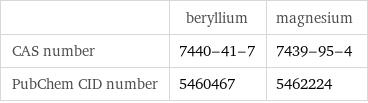
| beryllium | magnesium CAS number | 7440-41-7 | 7439-95-4 PubChem CID number | 5460467 | 5462224