Input interpretation
indium (chemical element)
Periodic table location
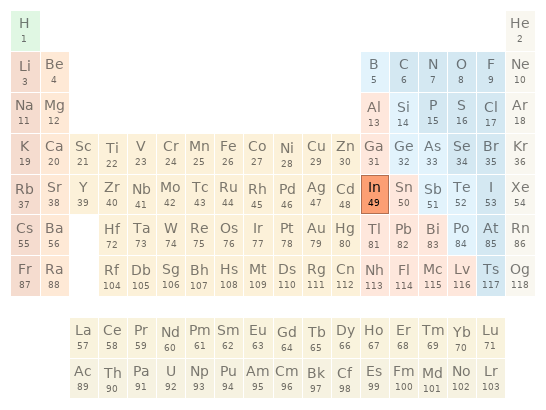
Periodic table location
Image
Image
Basic elemental properties
![atomic symbol | In atomic number | 49 short electronic configuration | [Kr]5s^24d^105p^1 Aufbau diagram | 5p 4d 5s block | p group | 13 period | 5 atomic mass | 114.818 u (unified atomic mass units)](../image_source/1ae8c3cc7a7c6b7e3e438d2e6edf5229.png)
atomic symbol | In atomic number | 49 short electronic configuration | [Kr]5s^24d^105p^1 Aufbau diagram | 5p 4d 5s block | p group | 13 period | 5 atomic mass | 114.818 u (unified atomic mass units)
Thermodynamic properties
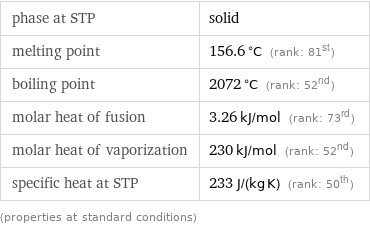
phase at STP | solid melting point | 156.6 °C (rank: 81st) boiling point | 2072 °C (rank: 52nd) molar heat of fusion | 3.26 kJ/mol (rank: 73rd) molar heat of vaporization | 230 kJ/mol (rank: 52nd) specific heat at STP | 233 J/(kg K) (rank: 50th) (properties at standard conditions)
Material properties
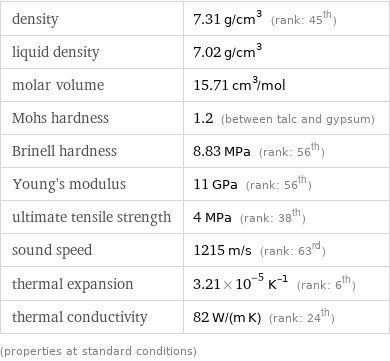
density | 7.31 g/cm^3 (rank: 45th) liquid density | 7.02 g/cm^3 molar volume | 15.71 cm^3/mol Mohs hardness | 1.2 (between talc and gypsum) Brinell hardness | 8.83 MPa (rank: 56th) Young's modulus | 11 GPa (rank: 56th) ultimate tensile strength | 4 MPa (rank: 38th) sound speed | 1215 m/s (rank: 63rd) thermal expansion | 3.21×10^-5 K^(-1) (rank: 6th) thermal conductivity | 82 W/(m K) (rank: 24th) (properties at standard conditions)
Electromagnetic properties
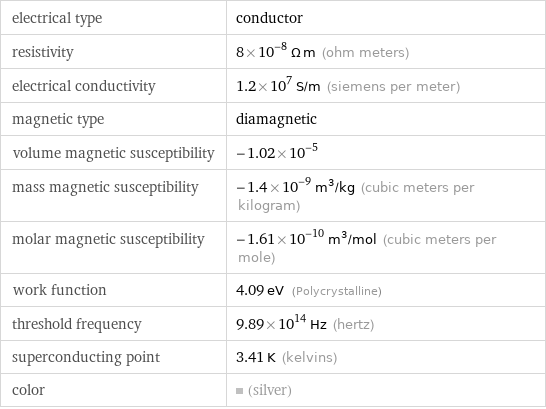
electrical type | conductor resistivity | 8×10^-8 Ω m (ohm meters) electrical conductivity | 1.2×10^7 S/m (siemens per meter) magnetic type | diamagnetic volume magnetic susceptibility | -1.02×10^-5 mass magnetic susceptibility | -1.4×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -1.61×10^-10 m^3/mol (cubic meters per mole) work function | 4.09 eV (Polycrystalline) threshold frequency | 9.89×10^14 Hz (hertz) superconducting point | 3.41 K (kelvins) color | (silver)
Reactivity
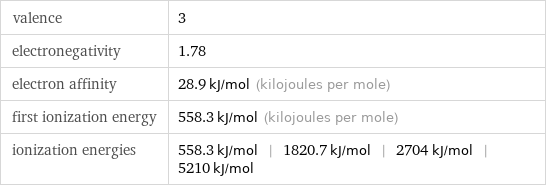
valence | 3 electronegativity | 1.78 electron affinity | 28.9 kJ/mol (kilojoules per mole) first ionization energy | 558.3 kJ/mol (kilojoules per mole) ionization energies | 558.3 kJ/mol | 1820.7 kJ/mol | 2704 kJ/mol | 5210 kJ/mol

Reactivity
Atomic properties
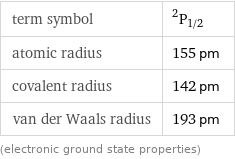
term symbol | ^2P_(1/2) atomic radius | 155 pm covalent radius | 142 pm van der Waals radius | 193 pm (electronic ground state properties)
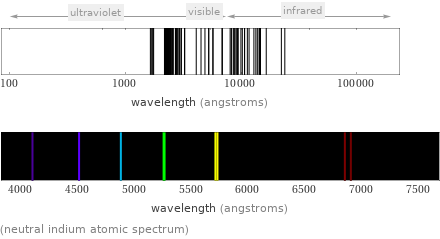
(neutral indium atomic spectrum)
Abundances

universe abundance | 3×10^-8 mass% (rank: 78th) solar abundance | 4×10^-7 mass% (rank: 44th) meteorite abundance | 4.4×10^-6 mass% (rank: 71st) crust abundance | 1.6×10^-5 mass% (rank: 65th) ocean abundance | 1×10^-11 mass% (rank: 76th) universe molar abundance | 3×10^-10 mol% (rank: 74th) solar molar abundance | 4×10^-9 mol% (rank: 46th) meteorite molar abundance | 1×10^-6 mol% (rank: 65th) ocean molar abundance | 5×10^-6 mol% (rank: 25th) crust molar abundance | 3×10^-6 mol% (rank: 68th)
Nuclear properties

half-life | (stable) stable isotopes | In-113 (4.29%) nuclear spin | In-97: 9/2^+ | In-98: 0^+ | In-99: 9/2^+ | In-101: 9/2^+ | In-102: 6^+ | In-103: 9/2^+ | In-104: 6^+ | In-105: 9/2^+ | In-106: 7^+ | In-107: 9/2^+ | In-108: 7^+ | In-109: 9/2^+ | In-110: 7^+ | In-111: 9/2^+ | In-112: 1^+ | In-113: 9/2^+ | In-114: 1^+ | In-115: 9/2^+ | In-116: 1^+ | In-117: 9/2^+ | In-118: 1^+ | In-119: 9/2^+ | In-120: 1^+ | In-121: 9/2^+ | In-122: 1^+ | In-123: 9/2^+ | In-124: 1^+ | In-125: 9/2^+ | In-126: 3^+ | In-127: 9/2^+ | In-128: 3^+ | In-129: 9/2^+ | In-130: 1^- | In-131: 9/2^+ | In-132: 7^- | In-133: 9/2^+ | In-135: 9/2^+ unstable isotopes | In-115 (441 trillion yr) | In-111 (67.3139 h) | In-110 (4.9 h) | In-109 (4.2 h) | In-108 (58 min) | In-117 (43.17 min) | In-107 (32.33 min) | In-112 (14.97 min) | In-106 (6.17 min) | In-105 (5.07 min) | In-119 (140 s) | In-104 (108 s) | In-114 (71.9 s) | In-103 (65 s) | In-102 (23.3 s) | In-121 (23.1 s) | In-101 (15.1 s) | In-116 (14.1 s) | In-123 (6.17 s) | In-100 (5.9 s) | In-118 (5 s) | In-124 (3.12 s) | In-120 (3.08 s) | In-99 (3 s) | In-125 (2.36 s) | In-126 (1.53 s) | In-122 (1.5 s) | In-127 (1.09 s) | In-128 (840 ms) | In-129 (611 ms) | In-130 (290 ms) | In-131 (280 ms) | In-132 (207 ms) | In-133 (165 ms) | In-134 (140 ms) | In-135 (92 ms) | In-98 (32 ms) | In-97 (5 ms) neutron cross-section | 194 b neutron mass absorption | 0.06 m^2/kg
Identifiers
CAS number | 7440-74-6 PubChem CID number | 5359967