Input interpretation

monoprotic acids | relative molecular mass
Summary
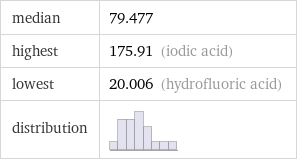
median | 79.477 highest | 175.91 (iodic acid) lowest | 20.006 (hydrofluoric acid) distribution |
Distribution plots
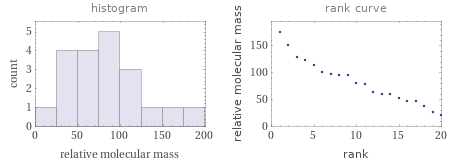
Relative molecular mass rankings
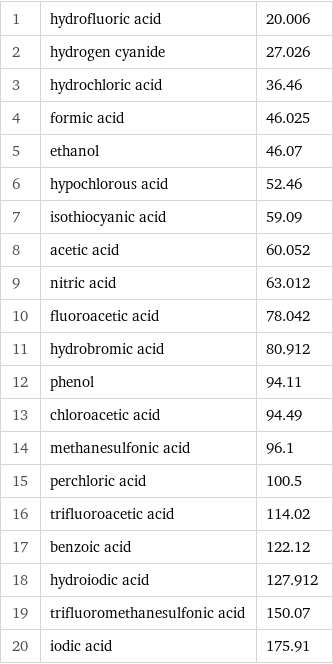
1 | hydrofluoric acid | 20.006 2 | hydrogen cyanide | 27.026 3 | hydrochloric acid | 36.46 4 | formic acid | 46.025 5 | ethanol | 46.07 6 | hypochlorous acid | 52.46 7 | isothiocyanic acid | 59.09 8 | acetic acid | 60.052 9 | nitric acid | 63.012 10 | fluoroacetic acid | 78.042 11 | hydrobromic acid | 80.912 12 | phenol | 94.11 13 | chloroacetic acid | 94.49 14 | methanesulfonic acid | 96.1 15 | perchloric acid | 100.5 16 | trifluoroacetic acid | 114.02 17 | benzoic acid | 122.12 18 | hydroiodic acid | 127.912 19 | trifluoromethanesulfonic acid | 150.07 20 | iodic acid | 175.91
Comparisons for median relative molecular mass 79.477

≈ ( 0.11 ≈ 1/9 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.41 × relative molecular mass of caffeine ( ≈ 194 )
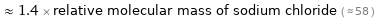
≈ 1.4 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
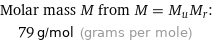
Molar mass M from M = M_uM_r: | 79 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 1.3×10^-22 grams | 1.3×10^-25 kg (kilograms)