Input interpretation
iron(II, III) oxide
Chemical names and formulas

formula | FeO·Fe_2O_3 Hill formula | Fe_3O_4 name | iron(II, III) oxide alternate names | black iron oxide | ferroso ferric oxide | ferrous ferric oxide | iron(II) diiron(III) oxide | magnetite mass fractions | Fe (iron) 72.4% | O (oxygen) 27.6%
Structure diagram
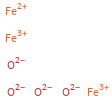
Structure diagram
Basic properties
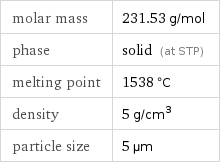
molar mass | 231.53 g/mol phase | solid (at STP) melting point | 1538 °C density | 5 g/cm^3 particle size | 5 µm
Solid properties (at STP)

density | 5 g/cm^3
Units

Thermodynamic properties
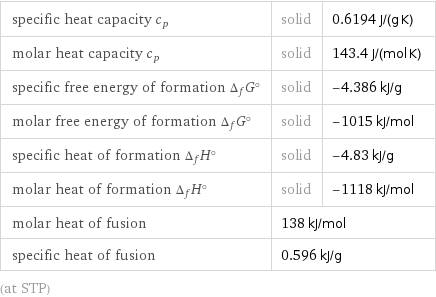
specific heat capacity c_p | solid | 0.6194 J/(g K) molar heat capacity c_p | solid | 143.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.386 kJ/g molar free energy of formation Δ_fG° | solid | -1015 kJ/mol specific heat of formation Δ_fH° | solid | -4.83 kJ/g molar heat of formation Δ_fH° | solid | -1118 kJ/mol molar heat of fusion | 138 kJ/mol | specific heat of fusion | 0.596 kJ/g | (at STP)
Chemical identifiers

CAS number | 1317-61-9 MDL number | MFCD00011010