Input interpretation
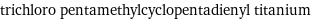
trichloro pentamethylcyclopentadienyl titanium
Basic properties
(C)(C)(C)(C1C=CC=C1)(Cl)(Cl)Cl InChI identifier | InChI=1/C5H5.5CH3.3ClH.Ti/c1-2-4-5-3-1;;;;;;;;;/h1-5H;5*1H3;3*1H;/q;;;;;;;;;+3/p-3/fC5H5.5CH3.3Cl.Ti/h;;;;;;3*1h;/q;;;;;;3*-1;m InChI key | WPFRCZFZOUFVAZ-UHFFFAOYSA-K](../image_source/705cc7268435d0b1d4d6dd60160fd5f7.png)
molar mass | 294.5 g/mol formula | C_10H_20Cl_3Ti empirical formula | Cl_3Ti_C_10H_20 SMILES identifier | C[Ti](C)(C)(C)(C)(C1C=CC=C1)(Cl)(Cl)Cl InChI identifier | InChI=1/C5H5.5CH3.3ClH.Ti/c1-2-4-5-3-1;;;;;;;;;/h1-5H;5*1H3;3*1H;/q;;;;;;;;;+3/p-3/fC5H5.5CH3.3Cl.Ti/h;;;;;;3*1h;/q;;;;;;3*-1;m InChI key | WPFRCZFZOUFVAZ-UHFFFAOYSA-K
Structure diagram
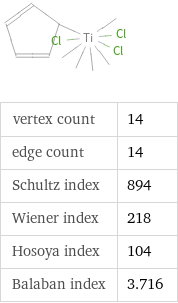
vertex count | 14 edge count | 14 Schultz index | 894 Wiener index | 218 Hosoya index | 104 Balaban index | 3.716
Quantitative molecular descriptors
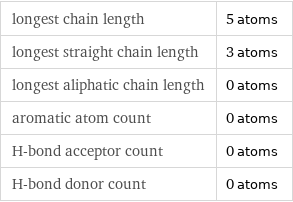
longest chain length | 5 atoms longest straight chain length | 3 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 0 atoms H-bond acceptor count | 0 atoms H-bond donor count | 0 atoms
Elemental composition
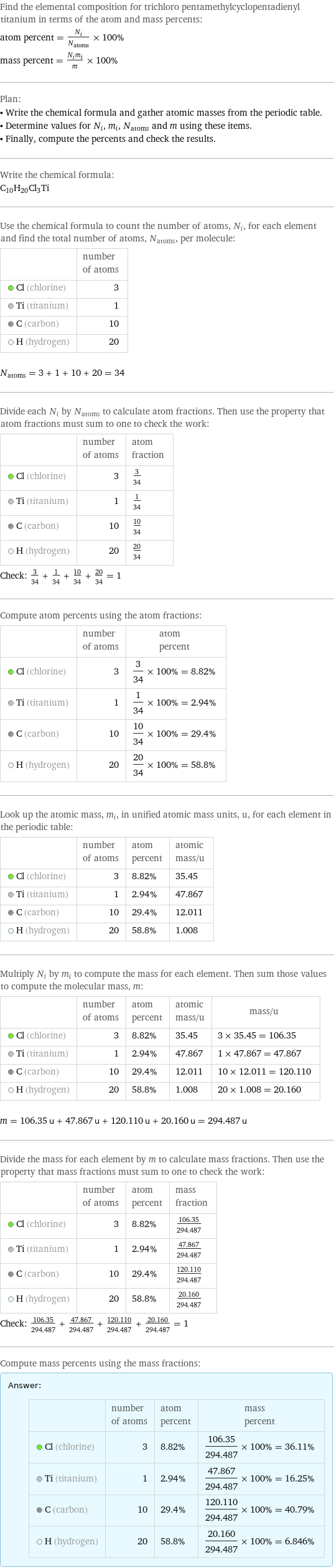
Find the elemental composition for trichloro pentamethylcyclopentadienyl titanium in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_10H_20Cl_3Ti Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms Cl (chlorine) | 3 Ti (titanium) | 1 C (carbon) | 10 H (hydrogen) | 20 N_atoms = 3 + 1 + 10 + 20 = 34 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction Cl (chlorine) | 3 | 3/34 Ti (titanium) | 1 | 1/34 C (carbon) | 10 | 10/34 H (hydrogen) | 20 | 20/34 Check: 3/34 + 1/34 + 10/34 + 20/34 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent Cl (chlorine) | 3 | 3/34 × 100% = 8.82% Ti (titanium) | 1 | 1/34 × 100% = 2.94% C (carbon) | 10 | 10/34 × 100% = 29.4% H (hydrogen) | 20 | 20/34 × 100% = 58.8% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u Cl (chlorine) | 3 | 8.82% | 35.45 Ti (titanium) | 1 | 2.94% | 47.867 C (carbon) | 10 | 29.4% | 12.011 H (hydrogen) | 20 | 58.8% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u Cl (chlorine) | 3 | 8.82% | 35.45 | 3 × 35.45 = 106.35 Ti (titanium) | 1 | 2.94% | 47.867 | 1 × 47.867 = 47.867 C (carbon) | 10 | 29.4% | 12.011 | 10 × 12.011 = 120.110 H (hydrogen) | 20 | 58.8% | 1.008 | 20 × 1.008 = 20.160 m = 106.35 u + 47.867 u + 120.110 u + 20.160 u = 294.487 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction Cl (chlorine) | 3 | 8.82% | 106.35/294.487 Ti (titanium) | 1 | 2.94% | 47.867/294.487 C (carbon) | 10 | 29.4% | 120.110/294.487 H (hydrogen) | 20 | 58.8% | 20.160/294.487 Check: 106.35/294.487 + 47.867/294.487 + 120.110/294.487 + 20.160/294.487 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent Cl (chlorine) | 3 | 8.82% | 106.35/294.487 × 100% = 36.11% Ti (titanium) | 1 | 2.94% | 47.867/294.487 × 100% = 16.25% C (carbon) | 10 | 29.4% | 120.110/294.487 × 100% = 40.79% H (hydrogen) | 20 | 58.8% | 20.160/294.487 × 100% = 6.846%
Topological indices
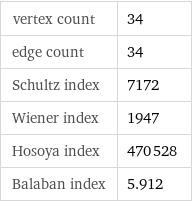
vertex count | 34 edge count | 34 Schultz index | 7172 Wiener index | 1947 Hosoya index | 470528 Balaban index | 5.912